Cellular mechanisms responsible for neurodevelopmental disorders
|
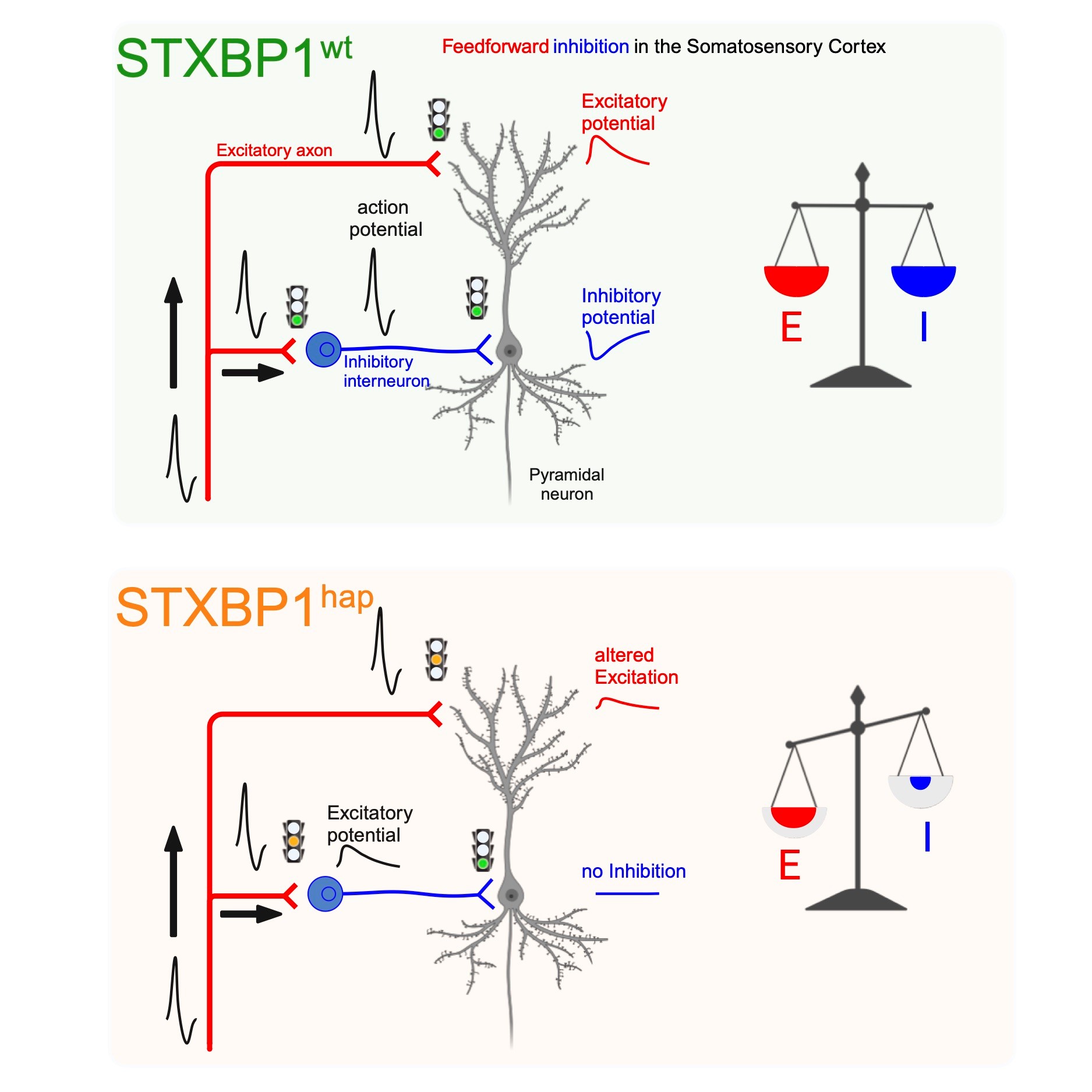
We are currently delving into how mutations identified in |
 |

Role of glial cells in motor controlWe are investigating how glial cells from the spinal cord contribute to motor control. We discovered that astrocytes from the ventral horn of the spinal cord respond to endocannabinoids released from active neighbor interneurons. In turn, astrocytes release purines which inhibit the release of excitatory neurotransmitters. This cellular mechanism acts as a filter that prevents motor tremor. It also explains in part the anti-tremor effect of cannabis reported by patients suffering from neurodegenerative diseases. See Carlsen et al., Nature Neuroscience 2021. |
Physiological function of neuronal intrinsic properties
Our lab has a developed a strong expertise in the study of intrinsic properties of individual neurons. By means of technique such as patch clamp recording, pharmacology, two-photon imaging in slice preparations from the central nervous, we are investigating how ion channels generate the electrical activity of neurons and how this contributes to the behavior of neural networks. Our recent results include:
 |
- The identification of the first cellular mechanism responsible for the motor fatigue that occurs in the central nervous system. Motoneurons are the final common output of the central nervous system. Their synaptic activation triggers the contraction of the muscle they innervate. Neurons from the raphe nuclei in the brainstem release serotonin on motoneurons by means of synaptic contacts. Their activation facilitates the activity of motoneurons and thereby muscle contraction. During intense physical activity, serotonin release is increased. A spillover occurs and serotonin reaches extrasynaptic receptors located on the axon initial segment of motoneurons. This inhibits action potential genesis and thereby muscle contraction. In that way serotonin prevents excessive muscle contraction (Cotel et al., 2013). |
 |
- The discovery of a new pathway that could prevent the development of temporal lobe epilepsy. The subiculum is a part of the temporal lope of the brain, and this is where temporal lobe epilepsy originates. Calcium flowing through CaV3 channels is responsible for the bursts of activity by pyramidal cells in the subiculum. When the activity of the neurons becomes overly synchronous, it results in abnormal electrical fluctuations, which lead to epileptic seizures. We have found that the activation of serotonin 2C receptors decreases the level of bursting by inhibiting CaV3 channels. This discovery could lead to the development of new principles for treating temporal lobe epilepsy (Petersen et al., 2017). See our popularization article in Videnskab.dk |
Quantum engineered magnetic field sensors
We are part of the Interfacing emerging quantum technology with biology and neurophysiology (BioQ) project initiated by the group of Ulrik Lund Andersen at the Technical University of Denmark. The objective of this Interdisciplinary Synergy Programme financed by NovoNordiskFonden is to image magnetic fields in brain tissue with resolutions from millimeter to nanometer-scale.
BioQ is a collaboration bringing together a dedicated team of quantum physicists and neurophysiologists from partners DTU Physics, DTU Electrical Engineering, Copenhagen University’s Department of Neuroscience and Hvidovre Hospital’s Danish Research Centre for Magnetic Resonance.
We recently obtained the first detection of the magnetic signal generated by a mammal muscle with a diamond sensor. See Webb et al. Scientific Reports volume 11, Article number: 2412 (2021)
 In collaboration with DTU, we will develop small, biocompatible and lightweight magnetic sensors with sensitivity suitable for mapping neuronal activity in living organisms. We recently demonstrated the ability of Nitrogen Vacancy centers to detect the magnetic field produced by action potentials propagating in neurons from the corpus callosum. See Hansen et al. Scientific Reports 2023.
In collaboration with DTU, we will develop small, biocompatible and lightweight magnetic sensors with sensitivity suitable for mapping neuronal activity in living organisms. We recently demonstrated the ability of Nitrogen Vacancy centers to detect the magnetic field produced by action potentials propagating in neurons from the corpus callosum. See Hansen et al. Scientific Reports 2023.
The project is funded by the Novo Nordisk Foundation Challenge Programme.
-
Dos Santos AB, Larsen SD, Guo L, Barbagallo P, Montalant A, Verhage M, Sørensen JB, Perrier JF. Microcircuit failure in STXBP1 encephalopathy leads to hyperexcitability. Cell Rep Med. 2023 Dec 7:101308. doi: 10.1016/j.xcrm.2023.101308.
-
Carlsen EM, Falk S, Skupio U, Robin L, Zottola AC, Marsicano G, Perrier JF. Spinal astroglial cannabinoid receptors control pathological tremor. Nature Neuroscience 2021 May;24(5):658-666.
-
Petersen AV, Cotel F, Perrier JF (2017). Plasticity of the axon initial segment: Fast and slow processes with multiple functional roles. The Neuroscientist, Volume: 23 issue: 4, page(s): 364-373.
-
Petersen AV, Jensen CS, Crépel V, Falkerslev M, Perrier JF (2017). Serotonin Regulates the Firing of Principal Cells of the Subiculum by Inhibiting a T-type Ca2+ Current. Frontiers in Cellular Neuroscience 11
- Christensen RK, Delgado-Lezama R, Russo RE, Lind BL, Loeza-Alcocer E, Rath R, Fabbiani G, Schmitt N, Lauritzen M, Petersen AV, Carlsen EM, Perrier JF (2018). Spinal dorsal horn astrocytes release GABA in response to synaptic activation. J Physiol, 596(20):4983-4994.
- Perrier JF, Cotel F (2015). Serotonergic modulation of spinal motor control. Current Opinion of Neurobiology, 33: 1-7.
- Cotel F, Exley R, Cragg SJ, Perrier JF (2013). Serotonin spillover onto the axon initial segment of motoneurons induces central fatigue by inhibiting action potential initiation. Proc Natl Acad Sci U S A. 110(12):4774-9.
-
Perrier JF, Hounsgaard J (2003) 5-HT2 receptors promote plateau potentials in turtle spinal motoneurons by facilitating an L-type calcium current. J Neurophysiol 89:954-959
-
Perrier JF, Hounsgaard J (2000) Development and regulation of response properties in spinal cord motoneurons. Brain Res Bull 53:529-535
Education
2015 Doctoral Dissertation at University of Copenhagen: Modulation of Motoneuron Activity by Serotonin
1996 PhD in Neuroscience at Université Pierre et Marie Curie (Paris, France).
1993 Master in Neuroscience at Université Pierre et Marie Curie (Paris, France).
Positions
Current position: Associate Professor Promotion Programme
Grants
The lab is funded by
- Novo Nordisk Fonden
- Danmarks Frie Forskningsråd
- Offerfonden
- Owensenske Fond
- Læge Sofus Carl Emil Friis og Hustru Olga Doris Friis' Legat
- Agnes og Poul Friis fond
- The Carlsberg Foundation
- Lundbeck Fondation
- Inge Berthelsen legat
- Antidoping Danmark
- Simon Fougner Harmanns Familiefond
- Danish Medical Research Council
Referee work
- Experimental Neurology
- British Journal of Pharmacology
- Cell and Tissue Research
- Cerebral Cortex
- European Journal of Neuroscience
- Frontiers
- Journal of Applied Physiology
- Journal of Comparative Neurology
- Journal of Neurophysiology
- Journal of Neuroscience
- Journal of Neuroscience Methods
- Journal of Physiology (London)
- Jove
- Life Sciences
- Nature Communications
- Neuroscience
- Physiology
Memberships of editorial boards and societies
- Society for Neurosciences (USA).
- Physiological Society (United Kingdom).
- Society for the Neural Control of Movement (USA).
- International Association for the Study of Pain.
- Hjerneforum
- Société des Neurosciences (France).
- Dansk Epilepsiforening
Talks (selection of recent invited lectures)
- STXPB1 encephalopathy: from basic to translational research. Synapse Biology in Health & Disease, Copenhagen 2025
- Exploring Neurodevelopmental Encephalopathies through STXBP1: Insights and Therapeutic Approaches. NeuroPSI – Institut des Neurosciences Paris-Saclay, France 2024
- Understanding and treating STXBP1 neurodevelopmental encephalopathy. 6th Dianalund International Conference on Epilepsy. Køge, Denmark, 2024
- Astrocytes provide the temporal dynamic required for memory formation in the hippocampus. 4th Nordic Neuroscience Meeting, Copenhagen, 2024
- Astrocytes provide the temporal dynamic required for memory formation in the hippocampus. Bonn Center of Neuroscience. Universität Bonn, Germany, 2023.
- STXPB1 encephalopathy: from basic to translational research. Emma Center for Personalized Medicine, Vrije Universiteit, Amsterdam, The Netherlands, 2023.
- Microcircuit failure in STXBP1 encephalopathy leads to hyperexcitability, rescue with AMPAkines. 1s t European STXBP1Summit and Research Roundtable. Milano, Italy, 2023.
- Understanding the cellular mechanisms responsible for neurodevelopmental encephalopathies. INCC seminar, Paris, France, 2023.
- Regulation of tremor by spinal astrocytes. XIV European Meeting on Glial Cells in Health and Disease. Porto 2019.
- Serotononergic control of motoneuron excitability. Lecture given for the graduate Course Understanding the Brain through the Hippocampus and other neural systems. Århus University, November 2017
- Role of endocannabinoids in the production of movement. 3rd INT neuroscience conference (in tribute to Laurent Vinay). Marseille, September 2016.
- Serotonin spillover onto the axon initial segment of motoneurons induces central fatigue by inhibiting action potential initiation. Department of Neurobiology Research, University of Southern Denmark, August 2016.
- Control of motor behavior by regulating the axon initial segment of motoneurons Brain Prize meeting. Middelfart, November 2015.
- Serotonergic control of motoneuron excitability. Seminaires de l’Institut du Fer à Moulin, Paris. October 2015.
- Serotonergic control of motoneuron excitability. International Neuroscience Symposium: “From ion channels to behaviour”. Copenhagen, June 2015.
- Serotonin spillover onto the axon initial segment of motoneurons induces central fatigue by inhibiting action potential initiation. Nordic Neuroscience Meeting, Trondheim, Norway, June 2015.
- Serotonin spillover onto the axon initial segment of motoneurons induces central fatigue by inhibiting action potential initiation. LVII National Meeting of the Mexican Physiological Society, Oaxca, Mexico, September 2014.
- Physiological role of the modulation of action potential generation at the axon initial segment. Physiology 2014. 07/2014 London.
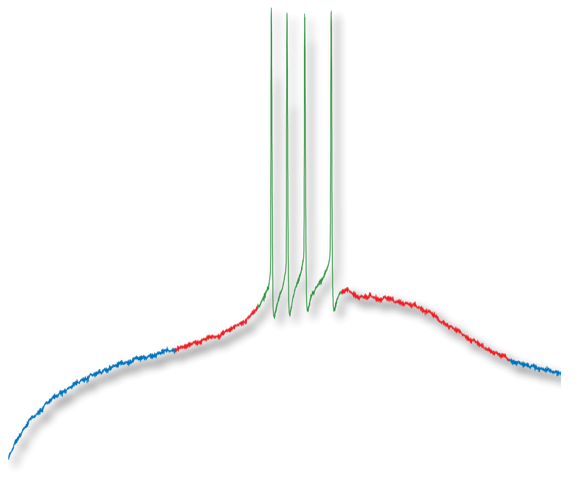
In vitro electrophysiology:
-
Intracellular recordings
-
Synaptic plasticity (short-term, LTP, LTD)
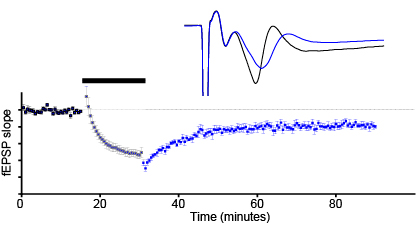
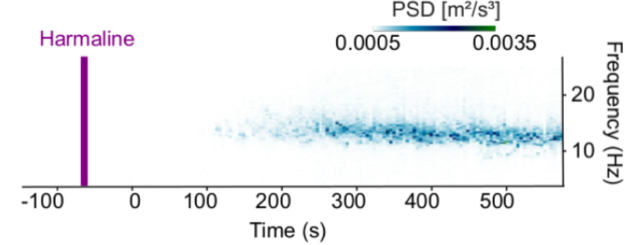
-
Tremor measurement
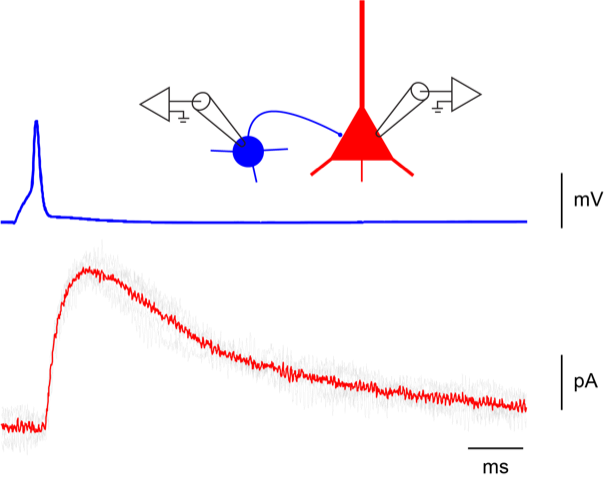
-
Patch-clamp (whole cell, outside-out)
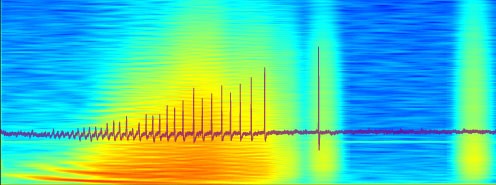
-
Local field potentials
-
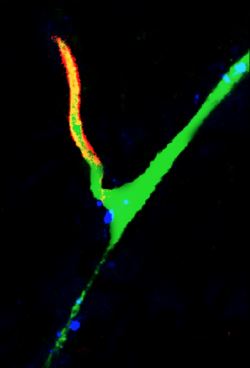
Imaging (multi-photon microscopy)
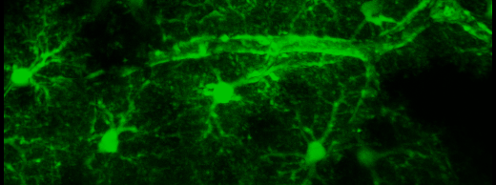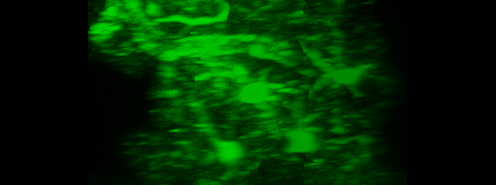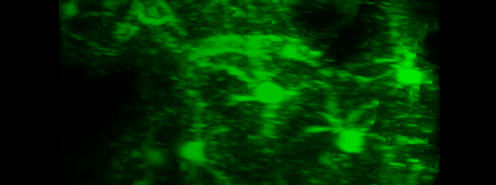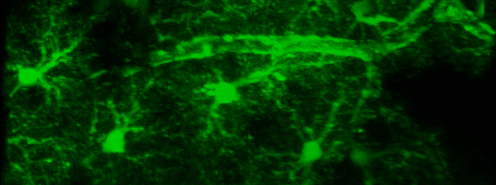
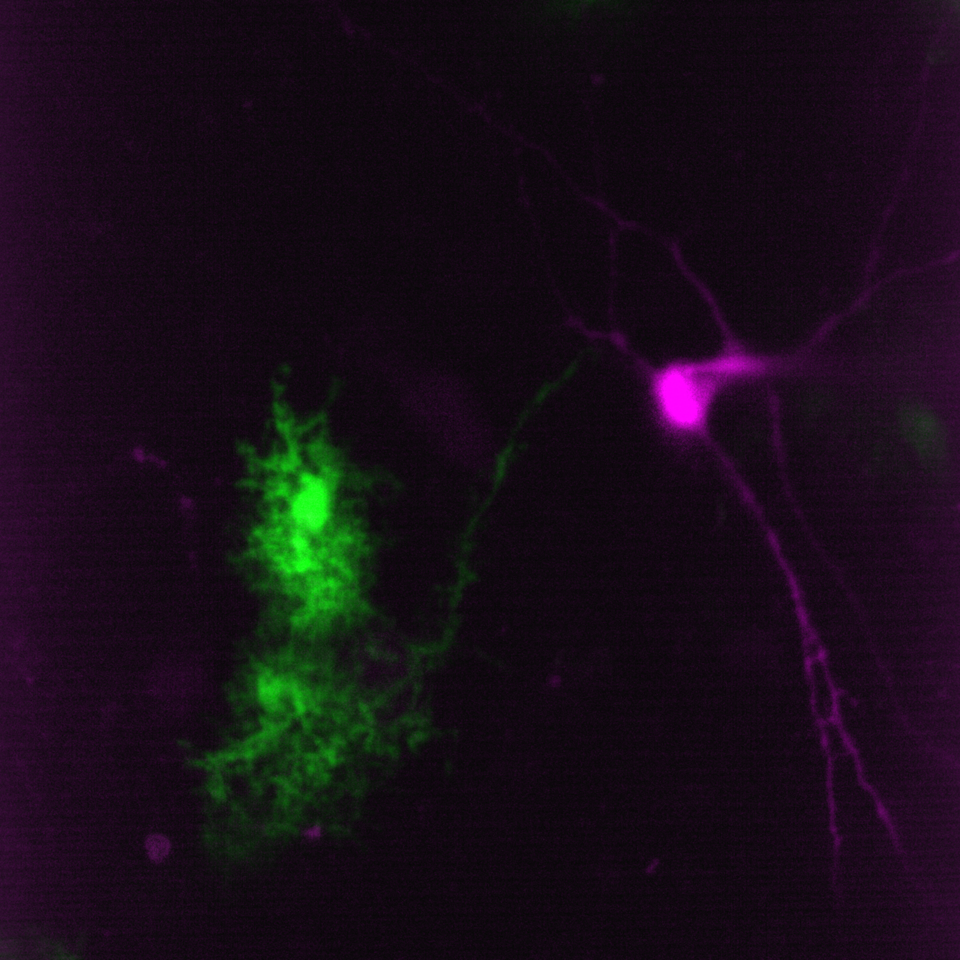
Simultaneous imaging of one neuron (purple) and two astrocytes (green) from the spinal cord. Credit: Alexia Montalant
-
Calcium imaging
Response of a spinal astrocyte to cannabinoid (Carlsen et al., 2021)
-
Optogenetics

-
Neuron Reconstruction
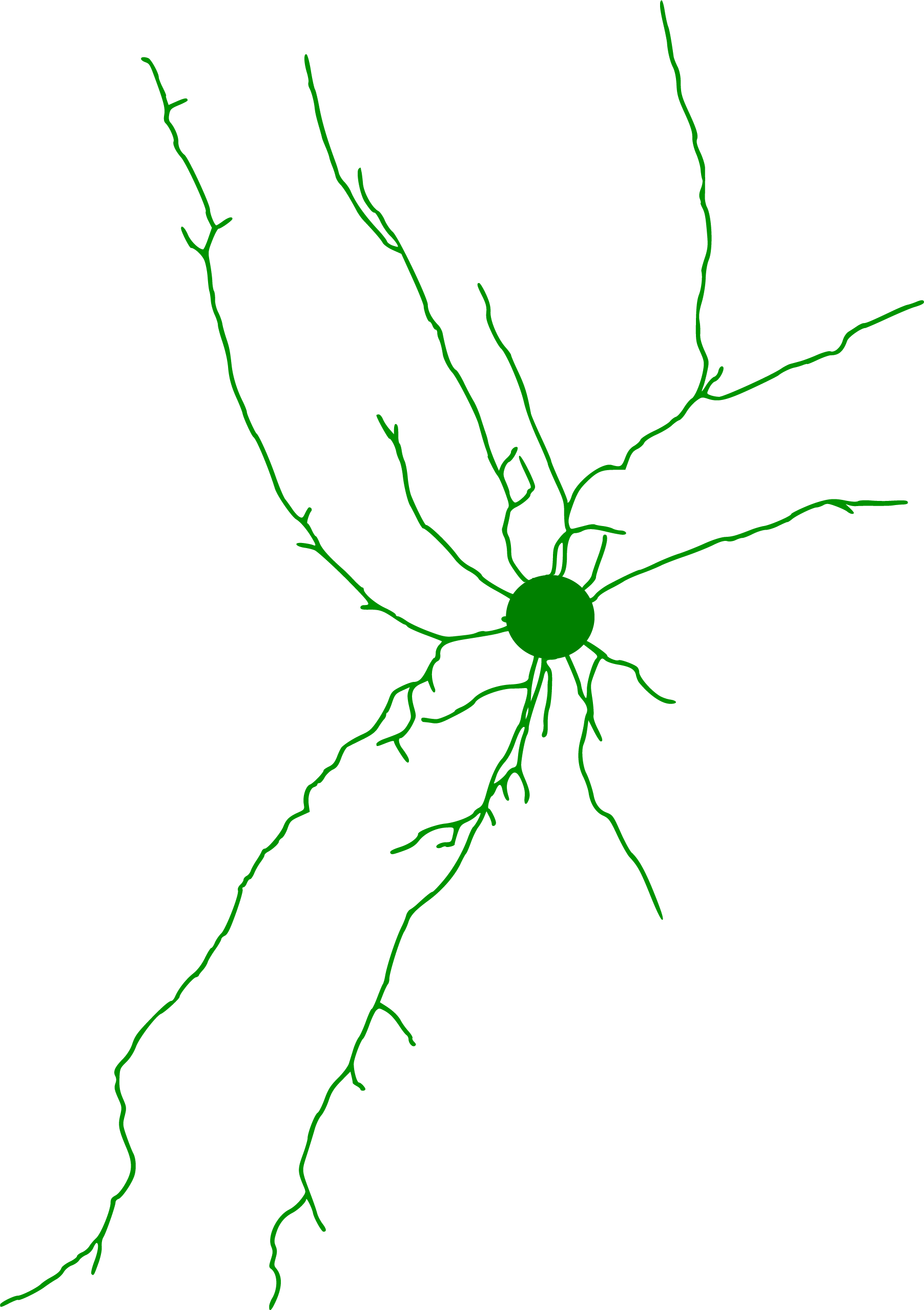
Credit: Victor Larsen
Course leader Bachelor module “Cellular Neuroscience”
Previous leader for Exitable Cells course (Bachelor in Medicine)
Teaching for Medical students (Bachelor), Biomedical engineering (Bachelor), Quantitative Biology and Disease Modelling (Bachelor), Human Biology (Bachelor, Master), Master of Neuroscience
- Carlos Daniel Gomez Martinez
- Hilligje Trijnie Gunnik
- Sarah Elisabeth Andersen
- Alexia Montalant
- Isabella Christiansen
- Eva Meier Carlsen, Postdoc at Center for Translational Neuromedicine, University of Copenhagen
- Dorhte Meinertz, retired
- Anders Victor Petersen, Head of supply chain development, A.P. Moller Mærsk
- Florence Cotel, Chairperson and Founder of BLISS
- Michelle Gjorlund, In-Field Medical Advisor at AbbVie
- Rasmus Kordt Christensen, Postdoc at University of Cambridge, UK
- Sarah Falk, Assistant Professor at Novo Nordisk Foundation Center for Basic Metabolic Research
- Sylvester Langvad, Chemist at Novo Nordisk
Publications in peer reviewed journals (lab members appear in bold)
67. Yan Y, Antolin N, Zhou L, Xu L, Vargas IL, Gomez CD, Kong G, Palmisano I, Yang Y, Chadwick J, Müller F, Bull AMJ, Lo Celso C, Primiano G, Servidei S, Perrier JF, Bellardita C, Di Giovanni S. Macrophages excite muscle spindles with glutamate to bolster locomotion. Nature. 2024 Dec 4. doi: 10.1038/s41586-024-08272-5.
66. Dos Santos AB, Larsen SD, Gomez CD, Sørensen JB, Perrier JF. Protocol for quantifying pyramidal neuron hyperexcitability in a mouse model of neurodevelopmental encephalopathy. STAR Protoc. 2024 Mar 15;5(2):102954.
65. Dos Santos AB, Larsen SD, Guo L, Barbagallo P, Montalant A, Verhage M, Sørensen JB, Perrier JF. Microcircuit failure in STXBP1 encephalopathy leads to hyperexcitability. Cell Rep Med. 2023 Dec 7:101308.
64. Montalant A, Kiehn O, Perrier JF. Dopamine and noradrenaline activate spinal astrocyte endfeet via D1-like receptors. Eur J Neurosci. 2023 Dec 5. doi: 10.1111/ejn.16205. Epub ahead of print. PMID: 38052454.
63. Hansen NW, Webb JL, Troise L, Olsson C, Tomasevic L, Brinza O, Achard J, Staacke R, Kieschnick M, Meijer J, Thielscher A, Siebner HR, Berg-Sørensen K, Perrier JF, Huck A, Andersen UL. Microscopic-scale magnetic recording of brain neuronal electrical activity using a diamond quantum sensor. Sci Rep. 2023 Jul 31;13(1):12407.
62. L. Troise, J. Webb, N. W. Hansen, C. Olsson, T. K. Pfau, L. Tomasevic, O. Brinza, J. Achard, R. Staacke, M. Kieschnick, J. Meijer, A. Thielscher, H. R. Siebner, J. Perrier, K. Berg-Sørensen, A. Huck, and U. L. Andersen, "Sensing of Biomagnetic Activity from Mammalian Tissues Using a Diamond Quantum Sensor," in Optical Sensors and Sensing Congress 2022 (AIS, LACSEA, Sensors, ES), Technical Digest Series (Optica Publishing Group, 2022), paper SM4C.2.
61. Troise L, Hansen NW, Olsson C, Webb JL, Tomasevic L, Achard J, Brinza O, Staacke R, Kieschnick M, Meijer J, Thielscher A, Siebner HR, Berg-Sørensen K, Perrier J-F, Huck A, Andersen UL (2022) In vitro recording of muscle activity induced by high intensity laser optogenetic stimulation using a diamond quantum biosensor. AVS Quantum Science 4:044402.
60. Webb JL, Troise L, Hansen NW, Frellsen LF, Osterkamp C, Jelezko F, Jankuhn S, Meijer J, Berg-Sørensen K,Perrier JF, Huck A, Andersen UL (2022). High-speed widefield imaging of microcircuitry using nitrogen vacancies in diamond. Phys. Rev. Applied, 17:064051
59. Montalant A, Carlsen EMM, Perrier JF. Role of astrocytes in rhythmic motor activity. Physiol Rep. 2021 Sep;9(18):e15029.
58. Magalhaes J, Ejlerskov P, Ering Hu E, Tresse E, Liu Y, Montalant A, Satriano L, Rundsten CF, Carlsen EM, Rydbirk R, Sharifi-Zarchi A, Andersen JB, Aznar S, Brudek T, Khodosevich K, Prinz M, Perrier JF, Sharma M, Gasser T, Marin A. PIAS2-mediated blockade of IFN-β signaling: A basis for sporadic Parkinson disease dementia. Mol Psychiatry. 2021 Jul 8. doi: 10.1038/s41380-021-01207.
57. Karadas M, Olsson C, Winther Hansen N, Perrier J-F, Webb JL, Huck A, Andersen UL and Thielscher A (2021) In-vitroRecordings of Neural Magnetic Activity From the Auditory Brainstem Using Color Centers in Diamond: A Simulation Study. Front. Neurosci.15:643614.
56. Carlsen EM, Falk S, Skupio U, Robin L, Zottola AC, Marsicano G, Perrier JF. Spinal astroglial cannabinoid receptors control pathological tremor. Nature Neuroscience 2021 May;24(5):658-666.
55. Webb JL, Troise L, Hansen NW, Olsson C, Wojciechowski AM, Achard J, Brinza O, Staacke R, Kieschnick M, Meijer J, Thielscher A, Perrier JF, Berg-Sørensen K, Huck A, Andersen UL. Detection of biological signals from a live mammalian muscle using an early stage diamond quantum sensor. Sci Rep. 2021 Jan 28;11(1):2412.
54. Webb JL, Troise L, Hansen NW, Achard J, Brinza O, Staacke R, Kieschnick M, Meijer J, Perrier J-F, Berg-Sørensen K, Huck A and Andersen UL (2020) Optimization of a Diamond Nitrogen Vacancy Centre Magnetometer for Sensing of Biological Signals. Front. Phys. 8:522536. doi: 10.3389/fphy.2020.522536
53. Sanz-Morello B, Pfisterer U, Winther Hansen N, Demharter S, Thakur A, Fujii K, Levitskii SA, Montalant A, Korshunova I, Mammen PP, Kamenski P, Noguchi S, Aldana BI, Hougaard KS, Perrier JF, Khodosevich K. Complex IV subunit isoform COX6A2 protects fast-spiking interneurons from oxidative stress and supports their function. EMBO J. 2020 Aug 3:e105759. doi: 10.15252/embj.2020105759
52. Carlsen E.M., Amrutkar D.V., Sandager K.N., Perrier, JF. (2019). Accurate and affordable assessment of physiological and pathological tremor in rodents with the accelerometer of a smartphone. J Neurophysiol. 122: 970–974, 2019.
51. Skov LJ, Ratner C, Hansen NW, Thompson JJ, Egerod KL, Burm H, Dalbøge LS, Hedegaard MA, Brakebusch C, Pers TH, Perrier JF, Holst B.. RhoA in tyrosine hydroxylase expressing neurons regulates food intake and body weight via altered sensitivity to peripheral hormones. J Neuroendocrinol. 2019 Jun 25. doi: 10.1111/jne.12761
50. Fitzpatrick CM, Runegaard AH, Christiansen SH, Jørgensen SH, Hansen NW, McGirr J, Sørensen AT, Perrier JF, Petersen A, Gether G, Woldbye DP, T Andreasen JT (2018). Differential effects of chemogenetic inhibition on dopamine and norepinephrine neurons in the mouse 5-choice serial reaction time task. Progress in Neuropsychopharmacology & Biological Psychiatry. Prog Neuropsychopharmacol Biol Psychiatry. 2019 Mar 2;90:264-276.
49. Perrier JF (2019). If serotonin does not exhaust you, it makes you stronger. J Physiol, 597(1):5-6.
48. Christensen RK, Delgado-Lezama R, Russo RE, Lind BL, Loeza-Alcocer E, Rath R, Fabbiani G, Schmitt N, Lauritzen M, Petersen AV, Carlsen EM, Perrier JF (2018). Spinal dorsal horn astrocytes release GABA in response to synaptic activation. J Physiol, 596(20):4983-4994.
47. Runegaard AH, Sørensen AT, Fitzpatrick CM, Jørgensen SH, Petersen A, Hansen NW, Weikop P, Andreasen JT, Mikkelsen JD, Perrier JF, Woldbye D, Rickhag M, Wortwein G and Gether U (2018). Locomotor- and reward-enhancing effects of cocaine are differentially regulated by chemogenetic stimulation of Gi-signaling in dopaminergic neurons. eNeuro, pii: ENEURO.0345-17.2018.
46. Perrier JF, Rasmussen H, Jørgensen, LK, Berg, RW (2018). Intense activity of the raphe spinal pathway depresses motor activity via a serotonin dependent mechanism. Front. Neural Circuits, 11:111. doi: 10.3389/fncir.2017.00111
45. von Schoubye NL, Frederiksen K, Kristiansen U, Petersen AV, Dalby NO, Morten Grunnet M, Jensen, HS, Jespersen T, Sohal VS, Perrier JF (2018). The sodium channel activator Lu AE98134 normalizes the altered firing properties of fast spiking interneurons in Dlx5/6+/- mice. Neuroscience Letters, 662, 29-35.
44. Petersen A and Perrier JF (2017). La sérotonine prévient l’épilepsie du lobe temporal en inhibant les neurones à bouffée du subiculum. Médecine Sciences, 33(8-9):727-729.
43. Gjørlund MD, Carlsen EM, Kønig AB, Dmytrieva O, Petersen A, Jacobsen J, Berezin V, Perrier J and Jacobsen SO (2017). Soluble ectodomain of neuroligin 1 decreases synaptic activity by activating metabotropic glutamate receptor 2. Front. Mol. Neurosci. 10:116. doi: 10.3389/fnmol.2017.00116
42. Petersen A, Jensen CS, Crépel V, Falkerslev M and Perrier JF (2017). Serotonin regulates the firing of principal cells of the subiculum by inhibiting a T-type Ca2+ current. Front. Cell. Neurosci. 11:60. doi: 10.3389/fncel.2017.00060
41. D'Amico JM, Annie A Butler AA, Heroux ME, Cotel F, Perrier JF, Butler J, Gandevia SC, and Janet L Taylor JL (2017). Human Motoneurone Excitability is Depressed by Activation of 5HT1A Receptors with Buspirone". J Physiol, 595, 1763-1773.
40. Carlsen EM, Rasmussen R. (2017). Bursting deep dorsal horn neurons: the pharmacological target for the antispastic effects of zolmitriptan? J Neurophysiol.;117(5):1841-1843.
39. Carlsen EM, Rasmussen R. (2017). Protein Networks in Alzheimer's Disease. Cell Syst. 4(2):153-155.
38. Rasmussen R, Carlsen EM. (2017). Motor cortical HCN channels: a contributor to coordinated forelimb movements in rodents? J Physiol. 595(3):633-634.
37. Petersen AV, Cotel F, Perrier JF (2017). Plasticity of the axon initial segment: Fast and slow processes with multiple functional roles. The Neuroscientist, Volume: 23 issue: 4, page(s): 364-373.
36. Rasmussen R, Carlsen EM. (2016). Spontaneous Functional Recovery from Incomplete Spinal Cord Injury. J Neurosci. 17;36(33):8535-7.
35. Perrier JF (2016). Modulation of motoneuron activity by serotonin. Dan Med J 2016:63(2);B5204
34. Benned-Jensen T, Christensen RK, Denti F, Perrier JF, Rasmussen H, and Olesen SP (2016). Live imaging of Kv7.2/7.3 cell surface dynamics at the axon initial segment: high steady state stability and calpain-dependent excitotoxic downregulation revealed. Journal of Neurosciences, 2631-15.
33. Petersen AV, Johansen EØ and Perrier J-F (2015) Fast and reliable identification of axons, axon initial segments and dendrites with local field potential recording. Front. Cell. Neurosci. 9:429
32. Korshunova Irina, Gjørlund MD, Owczarek S, Petersen AV, Perrier JF, Gøtzsche CR, Berezin V (2015). A neuroligin-1 derived peptide stimulates phosphorylation of the NMDA receptor subunit NR1 and rescues an MK-801 - induced decrease in LTP and memory impairment. Pharmacology Research & Perspectives, 3 (2) 1-12.
31. Perrier JF, Cotel F (2015). Serotonergic modulation of spinal motor control. Current Opinion of Neurobiology, 33: 1-7.
30. Carlsen EM, Perrier JF (2014). Purines released from astrocytes inhibit excitatory synaptic transmission in the ventral horn of the spinal cord. Front. Neural Circuits 8:60.
29. Christensen RK, Petersen AV, Schmitt N, Perrier JF (2014). Fast detection of extrasynaptic GABA with a whole-cell sniffer. Front. Cell. Neurosci. 8, 133.
28. Perrier JF (2013) Encadrement de l’excitabilité des motoneurones par la sérotonine. Médecine Sciences 29-6: 564-566.
27. Cotel F, Exley R, Cragg SJ, Perrier JF (2013) Serotonin spillover onto the axon initial segment of motoneurons induces central fatigue by inhibiting action potential initiation. Proceedings of the National Academy of Sciences of the United States of America 110:4774-4779.
26. Perrier JF, Rasmussen HB, Christensen RK, Petersen AV (2013) Modulation of the intrinsic properties of motoneurons by serotonin. Current pharmaceutical design 19: 4371-4384.
25. Christensen RK, Petersen AV, Perrier JF (2013) How do glial cells contribute to motor control? Current pharmaceutical design 19: 4385-4399.
24. Munch AS, Smith M, Moldovan M, Perrier JF (2010) Visual patch clamp recording of neurons in thick portions of the adult spinal cord. J Neurosci Methods 190:205-213.
23. Perrier JF, Cotel F (2008) Serotonin differentially modulates the intrinsic properties of spinal motoneurons from the adult turtle. J Physiol 586:1233-1238.
22. Smith M, Perrier JF (2006) Intrinsic properties shape the firing pattern of ventral horn interneurons from the spinal cord of the adult turtle. J Neurophysiol 96:2670-2677.
21. Perrier JF, Tresch MC (2005) Recruitment of motor neuronal persistent inward currents shapes withdrawal reflexes in the frog. J Physiol 562:507-520.
20. Perrier JF, Delgado-Lezama R (2005) Synaptic release of serotonin induced by stimulation of the raphe nucleus promotes plateau potentials in spinal motoneurons of the adult turtle. J Neurosci 25:7993-7999.
19. Grunnet M, Jespersen T, Perrier JF (2004) 5-HT1A receptors modulate small-conductance Ca2+-activated K+ channels. J Neurosci Res 78:845-854.
18. Simon M, Perrier JF, Hounsgaard J (2003) Subcellular distribution of L-type Ca2+ channels responsible for plateau potentials in motoneurons from the lumbar spinal cord of the turtle. Eur J Neurosci 18:258-266.
17. Perrier JF, Hounsgaard J (2003) 5-HT2 receptors promote plateau potentials in turtle spinal motoneurons by facilitating an L-type calcium current. J Neurophysiol 89:954-959.
16. Perrier JF, Alaburda A, Hounsgaard J (2003) 5-HT1A receptors increase excitability of spinal motoneurons by inhibiting a TASK-1-like K+ current in the adult turtle. J Physiol 548:485-492.
15. Perrier JF, Alaburda A, Hounsgaard J (2002) Spinal plasticity mediated by postsynaptic L-type Ca2+ channels. Brain Res Brain Res Rev 40:223-229.
14. Alaburda A, Perrier JF, Hounsgaard J (2002) Mechanisms causing plateau potentials in spinal motoneurones. Adv Exp Med Biol 508:219-226.
13. Alaburda A, Perrier JF, Hounsgaard J (2002) An M-like outward current regulates the excitability of spinal motoneurones in the adult turtle. J Physiol 540:875-881.
12. Perrier JF, Noraberg J, Simon M, Hounsgaard J (2000) Dedifferentiation of intrinsic response properties of motoneurons in organotypic cultures of the spinal cord of the adult turtle. Eur J Neurosci 12:2397-2404.
11. Perrier JF, Mejia-Gervacio S, Hounsgaard J (2000) Facilitation of plateau potentials in turtle motoneurones by a pathway dependent on calcium and calmodulin. J Physiol 528 Pt 1:107-113.
10. Perrier JF, Hounsgaard J (2000) Development and regulation of response properties in spinal cord motoneurons. Brain Res Bull 53:529-535.
9. Perrier JF, D'Incamps BL, Kouchtir-Devanne N, Jami L, Zytnicki D (2000) Cooperation of muscle and cutaneous afferents in the feedback of contraction to peroneal motoneurons. J Neurophysiol 83:3201-3208.
8. Perrier JF, D'Incamps BL, Kouchtir-Devanne N, Jami L, Zytnicki D (2000) Effects on peroneal motoneurons of cutaneous afferents activated by mechanical or electrical stimulations. J Neurophysiol 83:3209-3216.
7. Perrier JF, Hounsgaard J (1999) Ca(2+)-activated nonselective cationic current (I(CAN)) in turtle motoneurons. J Neurophysiol 82:730-735.
6. Delgado-Lezama R, Perrier JF, Hounsgaard J (1999) Local facilitation of plateau potentials in dendrites of turtle motoneurones by synaptic activation of metabotropic receptors. J Physiol 515 ( Pt 1):203-207.
5. Delgado-Lezama R, Perrier JF, Hounsgaard J (1999) Oscillatory interaction between dorsal root excitability and dorsal root potentials in the spinal cord of the turtle. Neuroscience 93:731-739.
4. Kouchtir N, Destombes J, Perrier JF, Horcholle-Bossavit G, Jami L (1997) Short-latency synaptic patterns among cat peroneal motoneurons. Brain Res 774:159-166.
3. Delgado-Lezama R, Perrier JF, Nedergaard S, Svirskis G, Hounsgaard J (1997) Metabotropic synaptic regulation of intrinsic response properties of turtle spinal motoneurones. J Physiol 504 ( Pt 1):97-102.
2. Zytnicki D, Lafleur J, Kouchtir N, Perrier JF (1995) Heterogeneity of contraction-induced effects in neurons of the cat dorsal spinocerebellar tract. J Physiol 487 ( Pt 3):761-772.
1. Kouchtir N, Perrier JF, Zytnicki D, Jami L (1995) Contraction-induced excitation in cat peroneal motoneurons. J Neurophysiol 73:974-982.
We are always looking for motivated candidates to join our research team (bachelor, Master, PhD or Postdoc).
If you are hard working and interested in neuroscience, please contact Jean-François Perrier (perrier@sund.ku.dk)


Lab members
| Name | Title | Job responsibilities | |
|---|---|---|---|
| Search in Name | Search in Title | Search in Job responsibilities | |
| Anna Christine Lera Nielsen | Laboratory Assistant | Perrier Lab |
|
| Anne Marie Nordvig Petersen | Laboratory Technician | Perrier Lab |
|
| Jean-Francois Marie Perrier | Professor | Perrier lab |
|
| Joakim Palmqvist | PhD Fellow | Perrier Lab |
|
| Jørn Dybkjær Hounsgaard | Professor Emeritus | Perrier Lab |
|
| Nanna Bülow Nøhr | Bachelor student | Perrier Lab |
|
| Niklas Mathias Valentin Jeske | Bachelor student | Perrier Lab |
|
| Nikolaj Winther Hansen | Postdoc | Perrier Lab |
|
| Victor Larsen | Laboratory Assistant | Perrier Lab |
|



 Follow us on Blue Sky
Follow us on Blue Sky SANIONA
SANIONA