Cai Lab
We focus on the molecular and cellular mechanism of brain vascular disease. We also develop neuroprosthetic technology for treatment of brain disorders.

Our lab is driven by two main questions: How does brain work? How to cure brain disease? We aim to employ cutting-edge research technologies to investigate the mechanism. We also aim to find therapeutic strategies combining pharmacology, electrophysiology and engineering.
Ongoing projects:
Microvessel dysfunction in brain ischemic stroke and reperfusion
Like all organs in human body, our brain receive oxygen and nutrients supplied by a complicated vascular network, particularly via microvessels, or so-called capillaries. An ischemic stroke occurs when blood flow in part of the brain is blocked. After just a few minutes, the starved brain cells begin to die. The standard treatment by the doctor is to give medication to break up the clot causing the stroke. However, more than 50% of stroke patients do not show any sign of clinical improvement even after successful reperfusion of big arteries, i.e. reperfusion injury. One of the important reasons is capillary clogging and constriction.
 |
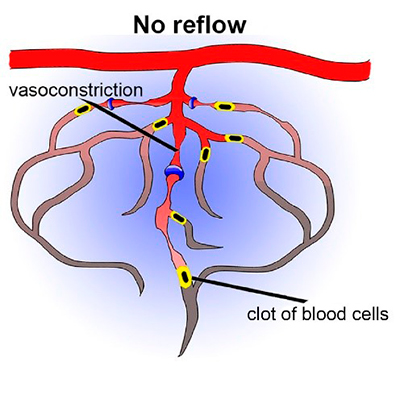 |
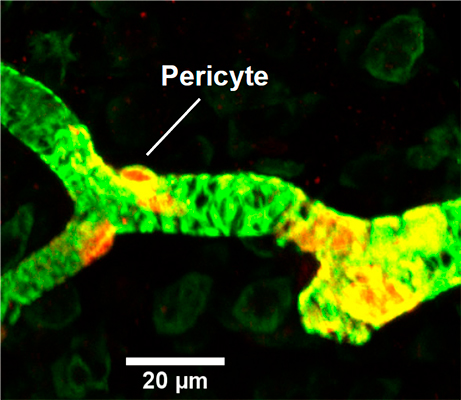
The aim of this project is to characterize the cellular mechanism responsible for reperfusion injury after an ischemic stroke and explore new vascular targets that potentially can be used to help stroke patients. The particular focus is on contractile mural cells on the wall of capillaries – pericytes.
Neuroprosthesis for vision restoration
WHO documents that over 60 million people worldwide are blind. Despite major advances in treatment, there are still patients who become blind due to the nerve disease. The recent advances in bionic technology and engineering enable us to build electronic devices, which act as a replacement for the failing part in the visual system. We are working on a bionic prosthesis on the visual cortex, to deliver stimulations based on the image-recording and digitalizing the external environment.
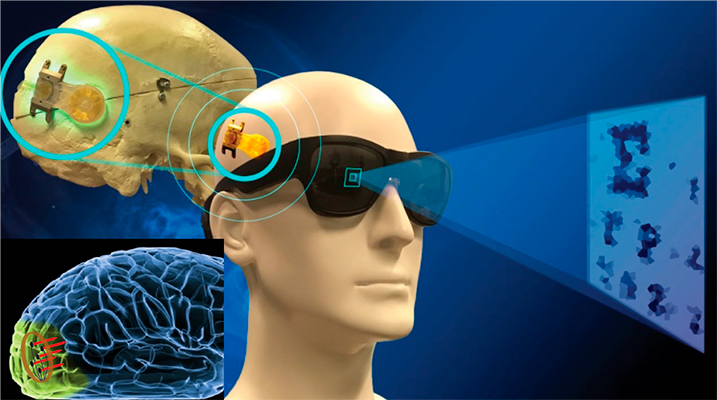
In collaboration with DTU and Harvard University, we are developing the next-generation cortical prosthesis device using miniature magnetic coils (microcoils). We further implant the microcoils in mouse visual cortex, and use state-of-the-art four-dimensional two-photon microscopy to study the long-term brain responses in awake mouse.
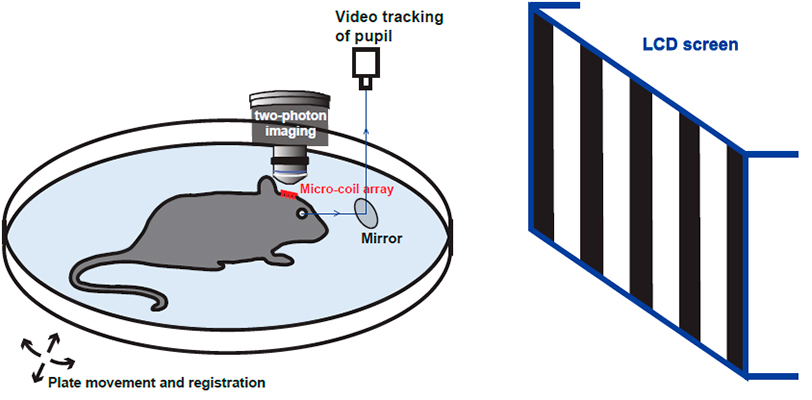 We aim to provide critical knowledge and technology toward the development of artificial vision and neuroprosthesis. We hope to provide the blind and their families with a much higher life quality, reduce healthcare costs to the whole society.
We aim to provide critical knowledge and technology toward the development of artificial vision and neuroprosthesis. We hope to provide the blind and their families with a much higher life quality, reduce healthcare costs to the whole society.
- Yonza A.K.I., Tao L., Zhang X., Postnov D., Kucharz K., Lind B., Asiminas A., Han A., Sonego V., Kim K.#, Cai C.#. Spatially and temporally mismatched blood flow and neuronal activity by high-intensity intracortical microstimulation. Brain Stimulation. (2025). (Accepted, in press) # Corresponding author.
- Kim K., Liu X., Chang B., Li G., Anand G, Genelioglu S., Yonza A.K.I., Whalen A., Berg R., Han A., Cai C. #. Micro-Coil Neuromodulation at Single-Cell and Circuit Levels for Inhibiting Natural Neuroactivity, Neutralizing Electric Neural Excitation and Suppressing Seizures. Advanced Science. (2025). (Accepted, in press) # Corresponding author.
- Zhang X., Tao L., Nygaard A., Dong Y., Hong X., Goddard C., He C., Postnov D., Allodi I., Lauritzen M., Cai C.# Aging alters calcium signaling in vascular mural cells and drives remodeling of neurovascular coupling in the awake brain. Journal of Cerebral Blood Flow and Metabolism. 2025. https://doi:10.1177/0271678X251320455 # Corresponding author.
- Cai, C. #, Zambach, S., Grubb, S., Tao L., He C., Lind B., Thomsen K., Zhang, X., Hald B., Nielsen R., Kim K., Devor A., Lønstrup M., Lauritzen M #. Impaired dynamics of precapillary sphincters and pericytes at first-order capillaries predict reduced neurovascular function in the aging mouse brain. Nature Aging (2023). https://doi.org/10.1038/s43587-022-00354-1. # Corresponding author.
- Zambach S.*, Cai C.*#, Helms H.C., Hald B., Dong Y., Fordsmann J.C., Nielsen R.M., Hu J., Lonstrup M., Brodin B., Lauritzen M.J.# Precapillary sphincters and pericytes at first-order capillaries as key regulators for brain capillary perfusion. Proceedings of the National Academy of Sciences (PNAS) USA 2021, 118 (26). doi:10.1073/pnas.2023749118. * Co-first author. # Corresponding author.
- Grubb S.*, Cai C.*, Hald B.*, Khennouf L., Jensen A., Murmu R., Fordsmann J., Zambach S., Lauritzen M. Precapillary sphincters maintain perfusion in the cerebral cortex. Nature Communication. 2020 Jan 20; 11(1):395. doi: 10.1038/s41467-020-14330-z. * Co-first author.
- Cai, C.,Zambach, S. A., Fordsmann, J. C., Lønstrup, M., Thomsen, K. J., Jensen, A. G., Lauritzen, M. In Vivo Three-Dimensional Two-Photon Microscopy to Study Conducted Vascular Responses by Local ATP Ejection Using a Glass Micro-Pipette. Journal of Visualized Experiments. (148), e59286, doi:10.3791/59286 (2019).
- Cai C., Fordsmann J. C., Jensen S. H., Gesslein B., Lonstrup M., Hald B. O., Zambach S. A., Brodin B., Lauritzen M. J. Stimulation-induced increases in cerebral blood flow and local capillary vasoconstriction depend on conducted vascular responses. Proceedings of the National Academy of Sciences (PNAS)USA 2018, 115(25): E5796-E5804.
- Cai, C., Twyford, P., & Fried, S. The response of retinal neurons to high-frequency stimulation. Journal of Neural Engineering. 2013, 10(3), 036009.
- Cai C., Ren Q., Desai N., Rizzo J., Fried S. Response variability to high rates of electric stimulation in retinal ganglion cells. Journal of neurophysiology. 2011, 106: 153–162
POSITIONS
| 2023 – present | Associate Professor, Department of Neuroscience, University of Copenhagen, Denmark |
| 2019 – 2023 | Assistant Professor, Department of Neuroscience, University of Copenhagen, Denmark |
| 2014 – 2019 | PostDoctoral fellow, Department of Neuroscience, University of Copenhagen, Denmark |
| 2012 – 2014 | PostDoctoral fellow, Faculty of Health, Aarhus University, Denmark |
| 2008 – 2010 | Research fellow, Department of Neurosurgery, Massachusetts General Hospital/ Harvard Medical School, Boston, MA, USA |
EDUCATION
| 2007 – 2011 | Ph.D., Biomedical Engineering, School of Biomedical Engineering, Shanghai Jiao Tong University (SJTU; Joint program with Harvard Medical School) |
| 2005 – 2007 | M.E. (Master of Engineering), Biomedical Engineering, Department of Biomedical Engineering, SJTU |
| 2002 – 2006 | B.S. (Bachelor of Science), Applied Mathematics, Department of Mathematics, SJTU |
| 2001 – 2005 | B.E. (Bachelor of Engineering) (Highest GPA), Biomedical Engineering, Department of Computer Science, SJTU |
FELLOWSHIPS & GRANTS
The lab is funded by
- Lundbeck Foundation Fellowship (10 million dkk)
- Novo Nordisk Foundation
- Danmarks Frie ForskningsFond
- Helse Fonden
- P. Møller Fonden
- Torben & Alice Frimodts Fond
- Augustinus Fonden
- Carl og Ellen Hertz Familielegat
- Oda og Hans Svenningsens Fond
REFEREE WORK
- Journal of Cerebral Blood Flow & Metabolism
- Cerebral Cortex
- IEEE Transactions on Biomedical Engineering
- Jove
SUPERVISION
Main project manager and co-supervisor of 3 PhD students and 2 Master students.
Two-photon microscopy in awake and behaving mice, as well as anesthetized mice are the main tool of our ongoing research projects. Our technical expertise includes:
◊ Mouse brain stroke and reperfusion model based on intraluminal filament
◊ Two-photon imaging microscopy
◊ Laser-speckle imaging
◊ Virus injection of genetically encoded calcium indicators
◊ Anesthetized and awake mouse brain studies
◊ Electrophysiology
◊ Immunohistochemistry
◊ Acute or chronic implantation of multi-electrode array in mouse brain
◊ MATLAB, Python and R programming for data analysis
Peer-reviewed journal articles
- Yonza A.K.I., Tao L., Zhang X., Postnov D., Kucharz K., Lind B., Asiminas A., Han A., Sonego V., Kim K.#, Cai C.#. Spatially and temporally mismatched blood flow and neuronal activity by high-intensity intracortical microstimulation. Brain Stimulation. (2025). (Accepted, in press) # Corresponding author.
- Kim K., Liu X., Chang B., Li G., Anand G, Genelioglu S., Yonza A.K.I., Whalen A., Berg R., Han A., Cai C. #. Micro-Coil Neuromodulation at Single-Cell and Circuit Levels for Inhibiting Natural Neuroactivity, Neutralizing Electric Neural Excitation and Suppressing Seizures. Advanced Science. (2025). (Accepted, in press) # Corresponding author.
- Zhang X., Tao L., Nygaard A., Dong Y., Hong X., Goddard C., He C., Postnov D., Allodi I., Lauritzen M., Cai C.# Aging alters calcium signaling in vascular mural cells and drives remodeling of neurovascular coupling in the awake brain. Journal of Cerebral Blood Flow and Metabolism. 2025. https://doi:10.1177/0271678X251320455 # Corresponding author.
- Cai, C. #, Zambach, S., Grubb, S., Tao L., He C., Lind B., Thomsen K., Zhang, X., Hald B., Nielsen R., Kim K., Devor A., Lønstrup M., Lauritzen M #. Impaired dynamics of precapillary sphincters and pericytes at first-order capillaries predict reduced neurovascular function in the aging mouse brain. Nature Aging (2023). https://doi.org/10.1038/s43587-022-00354-1. # Corresponding author.
- Cai, C., Lauritzen M. Changes in neurovascular function in brain microvessels during aging. Nature Aging (2023). https://doi.org/10.1038/s43587-022-00355-0.
- Zambach S.*, Cai C.*#, Helms H.C., Hald B., Dong Y., Fordsmann J.C., Nielsen R.M., Hu J., Lonstrup M., Brodin B., Lauritzen M.J. # Precapillary sphincters and pericytes at first-order capillaries as key regulators for brain capillary perfusion. Proceedings of the National Academy of Sciences (PNAS) USA 2021, 118 (26). doi:10.1073/pnas.2023749118. * Co-first author. # Corresponding author.
- Hørlyck S., Cai C., Helms H., Lauritzen M., Brodin B. ATP induces contraction of cultured brain capillary pericytes, via activation of P2Y type purinergic receptors. American Journal of Physiology - Heart and Circulatory Physiology. 2020 Dec. doi: 10.1152/ajpheart.00560.2020.
- Grubb S.*, Cai C.*, Hald B., Khennouf L., Jensen A., Murmu R., Fordsmann J., Zambach S., Lauritzen M. Precapillary sphincters maintain perfusion in the cerebral cortex. Nature Communication. 2020 Jan 20; 11(1):395. doi: 10.1038/s41467-020-14330-z. * Co-first author.
- Cai C., Fordsmann J. C., Jensen S. H., Gesslein B., Lonstrup M., Hald B. O., Zambach S. A., Brodin B., Lauritzen M. J. Stimulation-induced increases in cerebral blood flow and local capillary vasoconstriction depend on conducted vascular responses. Proceedings of the National Academy of Sciences (PNAS) USA 2018, 115(25): E5796-E5804.
- Cai, C., Zambach, S. A., Fordsmann, J. C., Lønstrup, M., Thomsen, K. J., Jensen, A. G., Lauritzen, M. In Vivo Three-Dimensional Two-Photon Microscopy to Study Conducted Vascular Responses by Local ATP Ejection Using a Glass Micro-Pipette. Journal of Visualized Experiments. (148), e59286, doi:10.3791/59286 (2019).
- Fordsmann J., Nielsen R., Cai C., Brazhe A., Thomsen K., Zambach S. A., Lonstrup M., Lind B. L., Lauritzen M. J. Spontaneous astrocytic Ca2+ activity abounds in electrically suppressed ischemic penumbra of aged mice. 2019. 67 (1), pp 37-52
- Nielsen, Fordsmann J., Cai C., Brazhe A., Thomsen K., Lauritzen M. Sensory Stimulation-Induced Astrocytic Calcium Signaling in Electrically Silent Ischemic Penumbra. Frontiers in Aging Neuroscience. 2019. 11(223), pp 1-12
- Fordsmann J., Nielsen R., Cai C., Brazhe A., Thomsen K., Zambach S. A., Lonstrup M., Lind B. L., Lauritzen M. J. Ion activity in mice offers insight into how to save stroke-stricken older brains. Research Square. 2019. doi:10.21203/rs.2.15069/v1
- Grubb S., Cai C., Khennouf L., Hald B., Murmu R., Zambach S., Lauritzen M. Precapillary sphincters exist in the brain and regulate blood flow to the capillary bed. Journal of Cerebral Blood Flow and Metabolism. 2019 (39).
- Cui K., Fan C., Chen G., Qiu Y., Li M., Lin M., Wan J., Cai C., Xiao Z. para-Aminothiophenol Radical Reaction-Functionalized Gold Nanoprobe for One-to-All Detection of Five Reactive Oxygen Species In Vivo. Analytical Chemistry. 2018, 90 (20), pp 12137–12144
- Jimenez E., Cai C., Mikkelsen I., Rasmussen P., Angleys H., Merrild M., Mouridsen K., Jespersen S., Lee J., Iversen N., Sakadzic S., Østergaard L. Effect of electrical forepaw stimulation on capillary transit-time heterogeneity (CTH). Journal of Cerebral Blood Flow and Metabolism. 2016, 36.12: 2072-2086.
- Khennouf L., Fordsmann J., Cai C., Brazhe A., Mathiesen C., Gesslein BV, Rasmussen Sondergaard, Kucharz K, Lauritzen M. The basis of vascular reactions during spreading depression and astroglial calcium waves. Journal of Cerebral Blood Flow and Metabolism. 2016, 36. 11:11
- Twyford P., Cai C., Fried S. Differential responses to high-frequency electrical stimulation in ON and OFF retinal ganglion cells. Journal of neural engineering. 2014, 11.2: 025001.
- Østergaard L., Aamand R, Karabegovic S, Tietze A, Blicher J, Mikkelsen I, Iversen N, Secher N, Engedal T, Anzabi M, Jimenez E., Cai C., Koch K, Naess-Schmidt E, Obel A, Juul N, Rasmussen M, Soerensen J. The role of the microcirculation in delayed cerebral ischemia and chronic degenerative changes after subarachnoid hemorrhage. Journal of Cerebral Flow and Metabolism. 2013, 33.12: 1825-1837.
- Cai, C., Twyford, P., & Fried, S. The response of retinal neurons to high-frequency stimulation. Journal of Neural Engineering. 2013, 10(3), 036009.
- Østergaard L., Jespersen S., Mouridsen K., Mikkelsen I., Jónsdottir K.Y., Tietze A., Blicher J., Hjort N., Iversen N.K., Cai C., Hovgaard K., Simonsen C., Von Weitzel-Mudersbach P., Modrau B., Nagenthiraja K., Ribe L., Hansen M., Bekke S., Dahlmann M., Puig J., Pedraza S., Serena J., Cho T.-H., Siemonsen S., Thomalla G., Fiehler J., Nighoghossian N., Andersen, G. The Role of the cerebral capillaries in acute ischemic stroke: The extended penumbra model. Journal of Cerebral Blood Flow & Metabolism. 2013, 33(5), 635-648.
- Cai C., Ren Q., Desai N., Rizzo J., Fried S. Response variability to high rates of electric stimulation in retinal ganglion cells. Journal of neurophysiology. 2011, 106: 153–162.
- Fried S., Cai C., Ren Q. High frequency electric stimulation of retinal neurons elicits physiological signaling patterns. 33rd Annual International Conference of the IEEE EMBS, 2011, Page: 1077-1080.
- Fried S., Cai C., Ren Q., Rizzo J. Factors affecting the reliability by which spikes are generated in response to high rates of stimulation. Investigative Ophthalmology & Visual Science. 52(14):4955-4956
- Sun J., Lu Y., Cao P., Li X., Cai C., Chai X., Ren Q., and Li L. Spatiotemporal Properties of Multi-peaked Electrically Evoked Potentials Elicited by Penetrative Optic Nerve Stimulation in Rabbits. Investigative Ophthalmology & Visual Science. 2011, 52(1): 146-154.
- Desai N.J., Rizzo J.F., Fried S.I., Cai C. Evaluation of Elicited Action Potential Kinetics in Response to Electric Stimulation of the Retina. Investigative Ophthalmology & Visual Science. 2010, 51(13):3045-3046
- Chen J., Doyle P., Cai C., Dumser J., Akhmechet R., Gingerich M.D., Kelly S.K., Shire D.B., Rizzo J.F. Surgical implantation of 1.5 generation retinal implant in minipig eyes. Investigative Ophthalmology & Visual Science. 51 (13), 3052-3052
- Cai C., Li L., Li X., Chai X., Sun J., Lu Y., Sui X., Chen P., Ren Q. Response properties of electrically evoked potential elicitesd by multi-channel penetrative optic nerve stimulation in rabbits. Documenta ophthalmologica. 2009, 118 (3): 191-204.
- Li X., Cai C., Li L., Chai X., Ren Q. Low-hemorrhage-risk surgical approach to expose the optic nerve in rabbits without bony removal and rectus resection. Veterinary ophthalmology. 2009, 12(4): 227-33
- Li L., Sun M., Cao P., Cai C., Chai X., Li X., Chen P. and Ren Q. A visual prosthesis based on optic nerve stimulation: In vivo electrophysiological study in rabbits. 7th Asian-Pacific Conference on Medical and Biological Engineering IFMBE Proceedings, 2008, Volume 19, Part 2, 54-57.
- Cai C., Zhao Y., Li L., Wu K., Chai X., Ren Q. Multi-channel microelectrode recording of MUA in cat visual cortex by electrical stimulation in optic nerve. 2007 IEEE/ICME International Conference on Complex Medical Engineering. 2007 (5): 1302-1306.
- Chai X., Yu W., Wang J., Zhao Y., Cai C., Ren Q. Recognition of pixelized Chinese characters using simulated prosthetic vision. Artificial organs. 2007 Mar; 31(3):175-82.
Patents
Twyford, P., Cai, C., Fried, S. System and method for selective neural activation using high-frequency electrical stimulation. (Q&B 125141.00925) [QBLLP-ACTIVE.FID37360969]. US Patent 9,737,711. (2017)
Lab members
| Name | Title | Job responsibilities | |
|---|---|---|---|
| Search in Name | Search in Title | Search in Job responsibilities | |
| Amogh Ashok Dyavangoudar | Guest Researcher | Cai Lab |
|
| Anpan Han | Guest Researcher | Cai Lab |
|
| Changsi Cai | Associate Professor | Cai lab |
|
| Christina Latt Fjorbak | PhD Fellow | Lauritzen Lab |
|
| Henry Gordon Sansom | Guest Researcher | Cai Lab |
|
| Kayeon Kim | Assistant Professor | Cai Lab |
|
| Lechan Tao | Postdoc | Cai Lab |
|
| Xiao Zhang | Postdoc | Cai Lab, Lauritzen Lab |
|