MacAulay Lab
The research group focuses on elucidation of the molecular mechanisms underlying water and ion homeostasis in the mammalian brain under both physiological and pathophysiological conditions.
News
500 mL fluid is secreted from our vasculature into our brains each day via the small brain structure called choroid plexus – and equally much drained out again.
On its way through the brain’s ventricular system, this fluid carries waste products and metabolites 🗑️ with it (not, however, necessarily via AQP4-dependent convective flow 🌊 through the parenchyma, as dictated by the glymphatic hypothesis, but simply as the only possible transport means from our active nerve cells and back into the circulation).
We just discovered that this secretory tissue that produces our brain fluids turned out to retain its structure and characteristic features, its gene expression profile, and its capacity for CSF secretion throughout the life span of healthy rats 🐭. It does so by an excessively high metabolism/oxygen consumption 🦾 (that dwarfs that of brain tissue, muscle, and other fluid transporting epithelia) that – contrasting that of brain tissue – also is kept up with aging in these rats.
Please see poem for the short version of our study and/or find the article in Nature Communications and/or the NotebookLM podcast version (20 min of easy listening and you will know all there is to know about CSF secretion in aging😉).
Our findings supports the importance of brain fluid secretion throughout life – and may suggest a potential secretory deficiency in neurodegenerative pathologies (which – if so – could potentially be modulated therapeutically 💊 in the future).
Thanks to all our amazing contributors across disciplines, departments, universities, and countries - what a great journey: Sara Diana Lolansen, Trine L. Toft, Søren Norge Andreassen, Marleen Trapp, Annarita Patrizi, Jens Velde Andersen, Blanca Aldana, Stine Meinild Lundby, Emil Westi, Eszter Olga Révész, Jonathan Wardman, Chiara Salio, Marco Sassoè-Pognetto, and Flemming Dela 🙏
Stay brain fit - keep secreting 🧠 💦 🦾
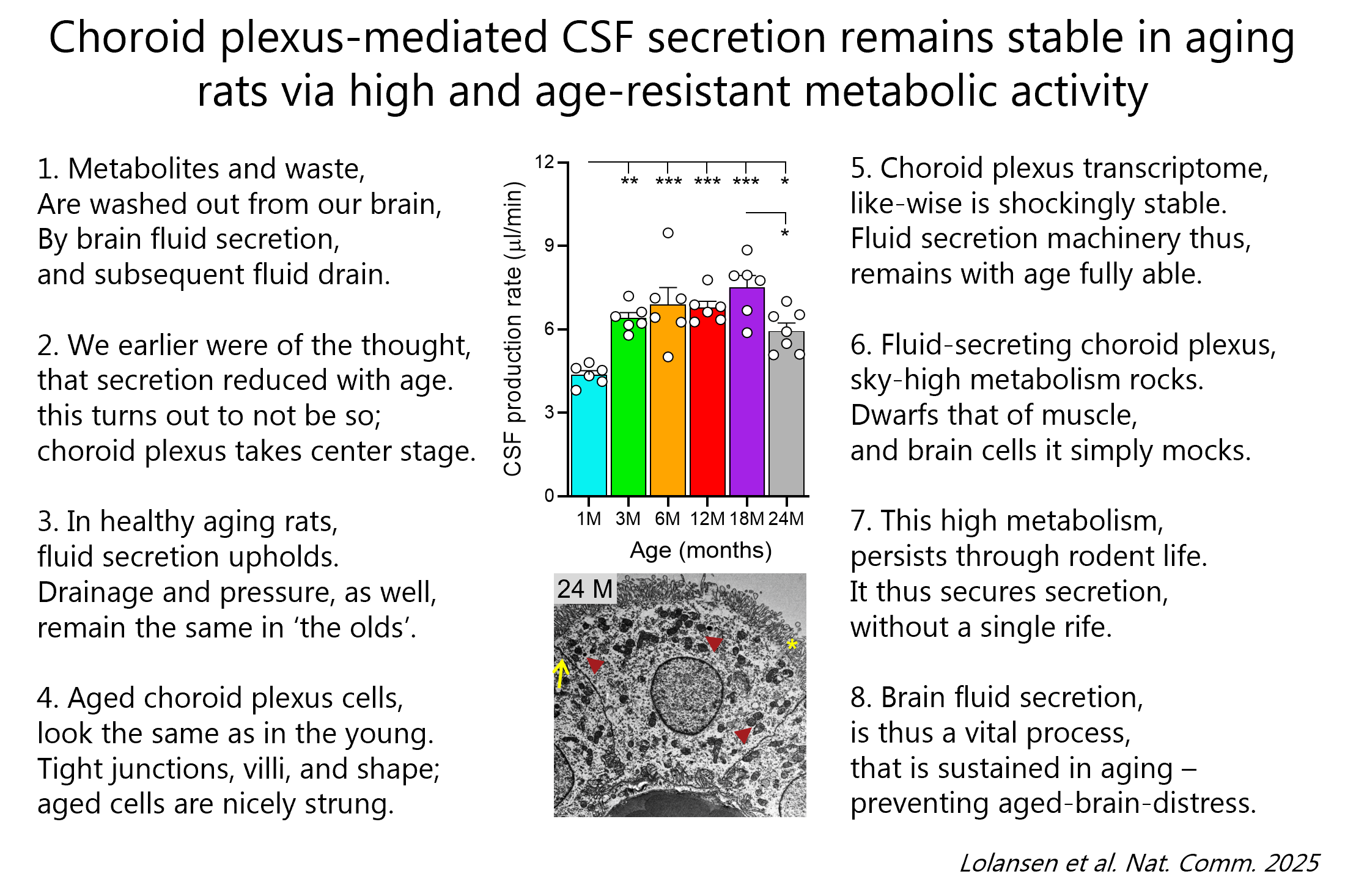
Rats and humans are same because both have increased brain fluid secretion at night (leading to elevated intracranial pressure), but different because humans sleep at night, while rats are awake (PMID: 37353833).
So, it is not sleep, per se, that controls the day/night brain fluid dynamics. It could be the vast changes in gene expression in the brain fluid-secreting tissue (the choroid plexus), in which as much as 20% of the genes fluctuate with the diurnal cycle (PMID: 37614671). Or, alternatively, as we just demonstrated (see science-poem for the short version or here (https://rdcu.be/epabH for the full article), the diurnal regulation of brain fluid secretion could be governed by the day/night brain-fluctuating neuromodulators noradrenalin and serotonin. These modulators increases during the day and repress the brain fluid secretion by acting on their receptors in the choroid plexus.
One more piece is now laid in the puzzle to delineate the molecular regulation of the brain fluid secretion apparatus.
Our vision remains focused on discovery of rational pharmacological targets in the regulatory pathways controlling the brain fluid secretion and thus the intracranial pressure.
Our aim is to lay the foundation for alternative and/or complementary treatment to the life-saving, but highly invasive, neurosurgical approaches employed in one form or another to treat hydrocephalus and other pressure-related neuropathologies since the stone age.
Thanks to my wonderful co-authors (Beatriche Edelbo, Annette B. Steffensen, Søren N. Andreassen, and Eszter Revesz) for their invaluable contributions and to the Lundbeck Foundation for their sustained support of our research.
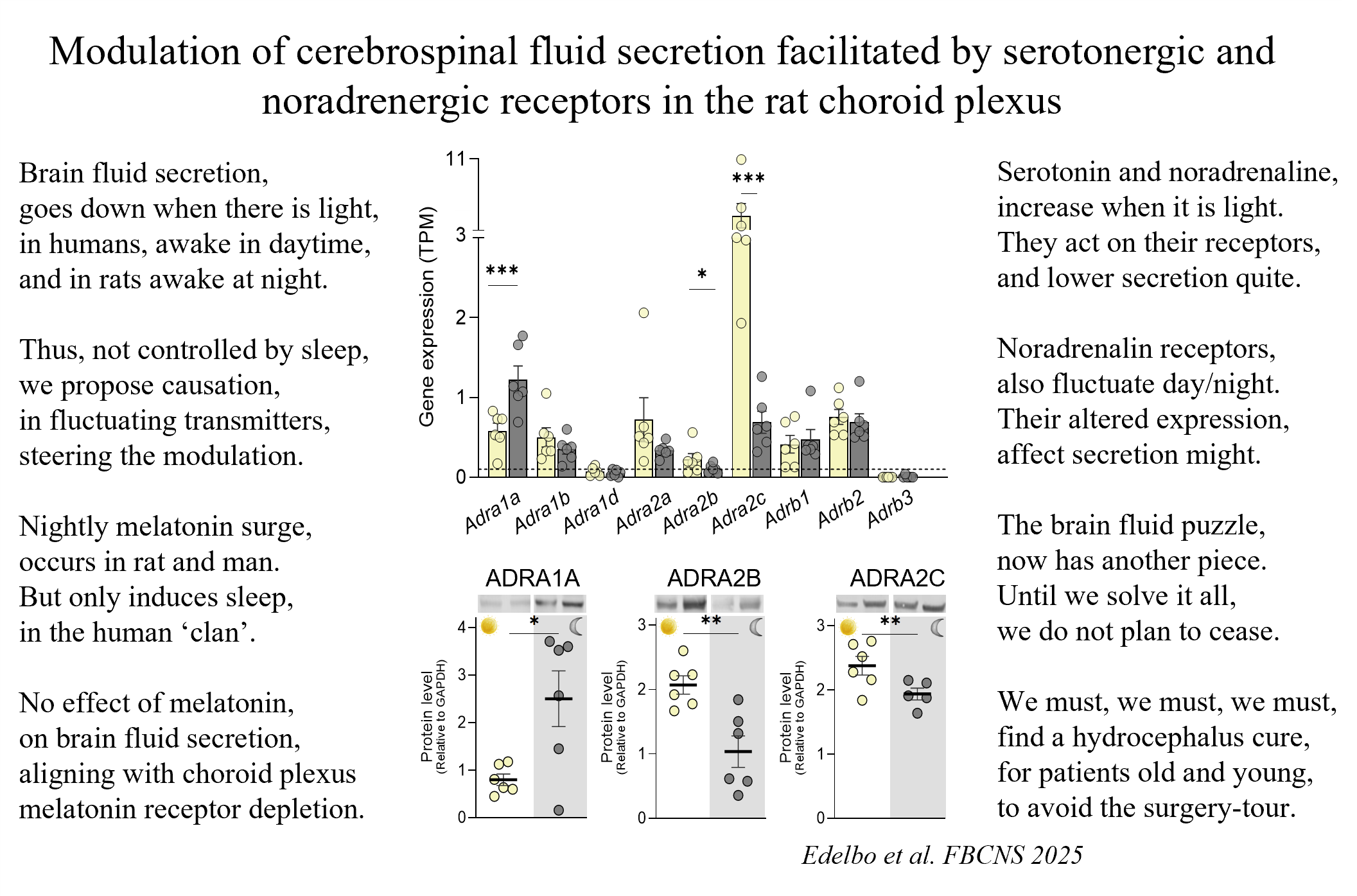
Never too much of a good thing (such as potassium, K+)
K+ is my absolutely favorite ion (don’t we all one?) Generally speaking, it is the ion that determines how much our neurons fire:
- a little extra and our neurons fire a bit more
- medium extra and our neurons go crazy (epilepsy, ischemia, migraine)
- much extra and our neurons go silent (cortical spreading depression).
K+ clearly must be very tightly regulated in the extracellular space in between our neurons and glia cells. The cellular origin (glia vs neurons) and the molecular mechanisms (the Na+/K+-ATPase versus Kir4.1-mediated spatial buffering) have been debated for more than half a century and my lab were keen contributors prior to us falling in love with the field of cerebrospinal fluid secretion, with our final piece just published in GLIA this week (see poem for the short version and here for the long version).
In short: the glial Na+/K+-ATPase of the α2β2 isoform combination senses the extracellular space increase in K+ that follows neuronal firing, and clears the excess K+ into the neighboring glia cells. These thus act as ‘sinks’ (holds on to the K+) until termination of the neuronal activity. K+ is then released back into the extracellular space, where it is taken up by the neuronal Na+/K+-ATPase of the α3β1 isoform combination, which is responsive to the elevated intracellular Na+ that occurs with the neuronal activity.
Voilá - the brain K+ shuttle is thereby complete. (with negligible quantitative contribution from the much-hyped Kir4.1 K+-channels).
This last piece has been lingering too long and it is fantastic to see it out in one of our favorite journals GLIA. Thanks to Brian Skriver Nielsen, Brian Roland Eriksen (Larsen), Afnan Bilal, Anca Stoica, K Brennan, and Steven Karlish for their contributions and discussion along the journey, and thanks to NIH and Novo Nordic Foundation for funding.
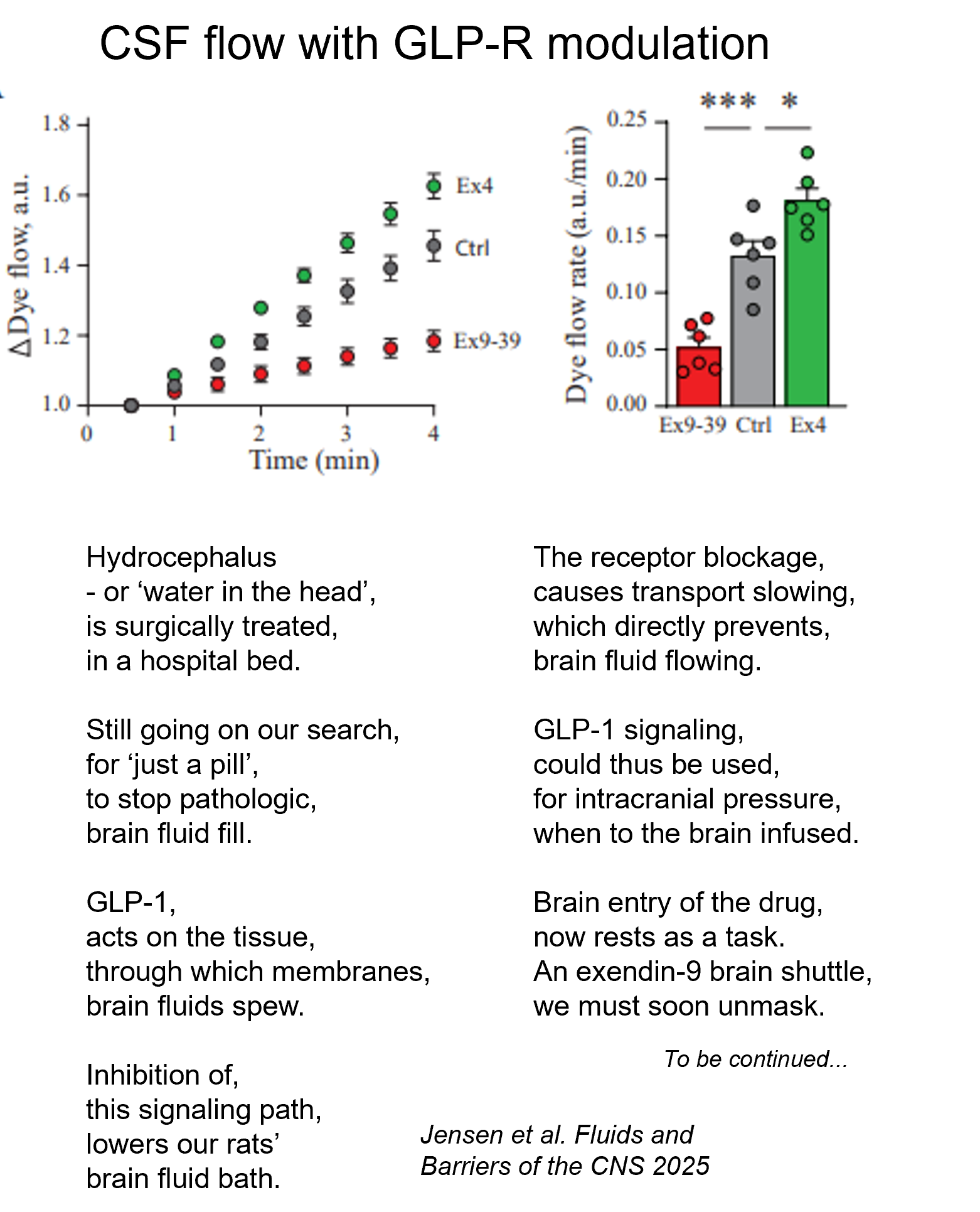
The peptide GLP-1, whose receptor is targeted by the various wonder-drugs (e.g. wegovy, victoza, ozempic – shout out to Novo Nordisk) as medication for T2-diabetes and obesity, can also control brain fluid secretion (in rats at least) by its action on the fluid-producing tissue called choroid plexus.
This tissue expresses low levels of the GLP-1 receptor. Activation of this receptor with administration of a GLP-1 receptor agonist (activator) directly into the brain of the experimental rats increased brain fluid secretion and oppositely with a GLP-1 receptor antagonist (inhibitor).
Check out our study just published for details (the short version as poetry).
Is therapeutic GLP-1 receptor modulation viable for treatment of the brain conditions characterized by too much fluid in the brain and thus elevated intracranial pressure? Maybe.
The first step would be to discover a way to get the GLP-1 receptor modulators across the brain barriers into the brain proper to reach their target in the fluid-secreting tissue. Why not target the brain-cerebrospinal fluid barrier itself – the choroid plexus? Possibly with a brain shuttle tag attached to the modulators…… Stay tuned….
Thanks to Mette Nyholm, Trine Toft-Bertelsen, Ida Israelsen, and Jonathan Wardman for their effort in my lab, to Mette Rosenkilde and Jens Juul Holst for their GLP-1 contributions, and to the Novo Nordisk foundation for their support.
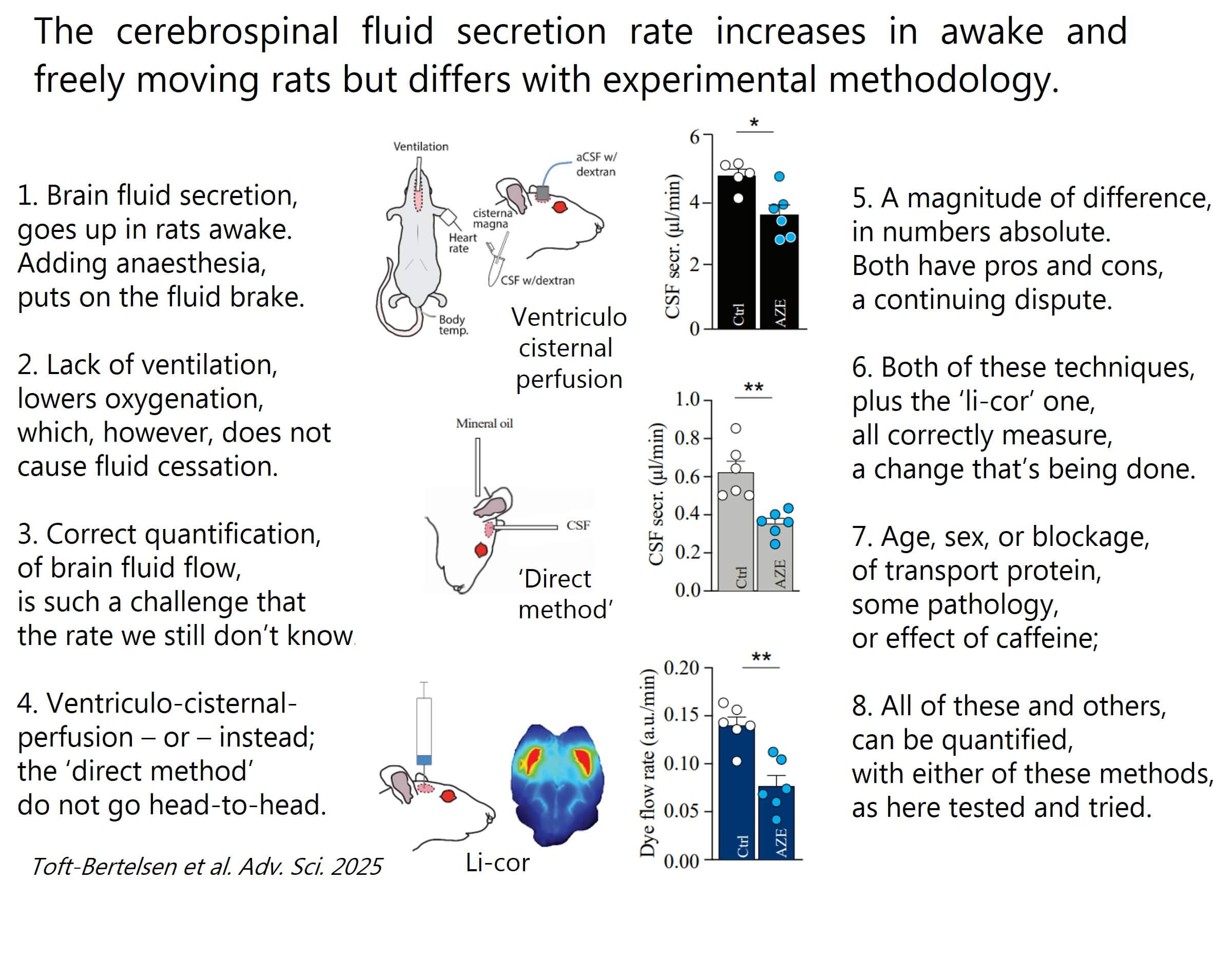
It is so challenging to measure brain fluid production.
To modulate the brain fluid content in pathologies with elevated brain fluid content, such as hydrocephalus of various kinds, we must know whether components of the underlying etiology originate from altered rates of brain fluid production. We therefore shall quantify changes in this rate correctly – starting with experimental animals in our newly published paper (see content on verse in this post or find a link to the paper in the comments below).
We find:
The rate of brain fluid production is higher in awake animals than when under anaesthesia
The brain fluid production rate (in absolute numbers) differs with the employed methodology
Three different methodologies accurately reflect a change in brain fluid production upon pharmacological modulation of the fluid-secreting tissue
Great news to all brain fluid afficionados – choose your favourite method and quantify brain fluid production with pathology, age, sex, inflammation pharmacological modulation, transport protein knockin/out, etc.
There is so much we still do not know – the field needs all hands on board!
Thanks to the Lundbeck Foundation for their support, to Trine for driving this project, and to our co-authors for their valuable contributions.
Nanna MacAulay researches the large quantity of water in the mammalian brain which is continuously shifted between the circulating blood and the brain parenchyma as well as between different compartments and cellular structures within the brain tissue. One presumes that the transport of water between these different compartments is under tight control since a disturbance in the cerebral water homeostasis (with associated changes in ion concentrations) may lead to neuronal dysfunction, hydrocephalus, and/or brain edema. However, the incomplete knowledge of the molecular mechanisms responsible for the maintenance of cerebral water transport and their regulation currently prevents the research field from gaining a full understanding of this intricate and crucial (patho)physiological issue. With this lack of identification of the implicated transport mechanisms and their dysregulation in pathology, pharmacological therapy is essentially unavailable for potentially life-threatening conditions involving brain water accumulation, i.e. hydrocephalus, brain edema, acute liver failure, idiopathic intracranial hypertension, etc.
The research group focuses on elucidation of the molecular mechanisms underlying water and ion homeostasis in the mammalian brain under both physiological and pathophysiological conditions. More specifically, the laboratory investigates the transport mechanisms underlying cerebrospinal fluid secretion with a technical approach spanning from molecular and biophysical properties of water transport proteins (including aquaporins and cotransporters) to their regulation at the cellular level and their integral function in rodent in vivo models of physiology and pathophysiology. Through extensive collaboration with the clinic, the laboratory performs CSF analysis of patient samples to elucidate the underlying etiology of pathologies such as posthemorrhagic hydrocephalus, normal pressure hydrocephalus, and obstructive hydrocephalus.
- Sara D. Lolansen, Eszter O. Révész, Søren N. Andreassen, Marleen Trapp, Chiara Salio, Marco Sassoé-Pognetto, Jens Velde Andersen, Emil W. Westi, Trine L. Toft-Bertelsen, Jonathan H. Wardman, Anne-Kristine Meinild Lundby, Flemming Dela, Annarita Patrizi, Blanca I. Aldana & Nanna MacAulay (2025) Choroid plexus-mediated CSF secretion remains stable in aging rats via high and age-resistant metabolic activity. Nature Communications accepted for publication.
- Trine L. Toft-Bertelsen, Beatriche L. Edelbo, Annette B. Steffensen, Sara D. Lolansen, Jonathan H. Wardman, Dennis B. Jensen & NannaMacAulay (2025) The cerebrospinal fluid secretion rate increases in awake and freely moving rats but differs with experimental methodology. Advanced Science, e2412469
- Dennis B. Jensen, Trine L. Toft-Bertelsen, Dagne Barbuskaite, Jane Stubbe, Sandor Nietzsche, Tenna Capion, Nicolas H. Norager, Markus H. Olsen, Andreas T. Sørensen, Henrik Dimke, Christian A. Hübner, Marianne Juhler & Nanna MacAulay (2025) The Na+,K+,2Cl-cotransporter, not aquaporin 1, sustains cerebrospinal fluid secretion while controlling brain K+ Advanced Science, e2409120
- Kristopher T. Kahle, Petra M. Klinge, Jenna E. Koschnitzky, AV Kulkarni, NannaMacAulay, S Robinson, SJ Schiff SJ & Jennifer M. Strahle (2024) Paediatric hydrocephalus. Nature Reviews Disease Primers 10:35
- Sara D. Lolansen, Nina Rostgaard, Tenna Capion, Nicolas H. Nørager, Markus H. Olsen, Marianne Juhler, Tiit I. Mathiesen & Nanna MacAulay(2023) Posthemorrhagic hydrocephalus in patients with subarachnoid hemorrhage occurs independently of CSF osmolality. International Journal of Molecular Science 24:11476
- Annette B. Steffensen, Beatriche L. Edelbo, Dagne Barbuskaite, Søren N. Andreassen, Markus H. Olsen, Kirsten Møller & Nanna MacAulay(2023) Nocturnal increase in cerebrospinal fluid secretion as a circadian regulator of intracranial pressure. Fluids and Barriers of the CNS 20:49
- Jonathan H. Wardman, Mette Nyholm Jensen, Søren Norge Andreassen, Bjarne Styrishave, Jens E. Wilhjelm, Alexandra J. Sinclair & Nanna MacAulay(2023) Modelling idiopathic intracranial hypertension in rats: contributions of high fat diet and testosterone to intracranial pressure and cerebrospinal fluid production. Fluids and Barriers of the CNS 20:44
- Trine L Toft-Bertelsen, Dagne Barbuskaite, Eva K Heerfordt, Sara D Lolansen, Søren N Andreassen, Nina Rostgaard, Markus H Olsen, Nicolas H. Norager, Tenna Capion, Martin F Rath, Marianne Juhler & Nanna MacAulay(2022) Lysophosphatidic acid as a CSF lipid in posthemorrhagic hydrocephalus that drives CSF accumulation via TRPV4-induced hyperactivation of NKCC1. Fluids and Barriers of the CNS 19:69
- Eva K. Oernbo, Annette B. Steffensen, Pooya R Khamesi, Trine L Toft-Bertelsen, Dagne Barbuskaite, Frederik Vilhardt, Niklas J. Gerkau, Katerina Tritsaris, Anja H Simonsen, Sara D Lolansen, Søren N Andreassen, Steen G. Hasselbalch, Thomas Zeuthen, Christine R. Rose, Vartan Kurtcuoglu & Nanna MacAulay(2022) Membrane transporters control cerebrospinal fluid formation independently of conventional osmosis to modulate intracranial pressure. Fluids and Barriers of the CNS 19:65
- Sara D. Lolansen, Nina Rostgaard, Dagne Barbuskaite, Tenna Capion, Markus H. Olsen, Nicolas H. Norager, Frederik Vilhardt, Søren N. Andreassen, Trine L. Toft-Bertelsen, F. Ye, Marianne Juhler, Richard Keep & Nanna MacAulay (2022) Posthemorrhagic hydrocephalus associates with elevated inflammation and CSF hypersecretion via activation of choroidal transporters. Fluids and Barriers of the CNS 19:62
- Dagne Barbuskaite, Eva K. Oernbo, Jonathan H. Wardman, Trine L. Toft-Bertelsen, Eller Conti, Søren N. Andreassen, Niklas J. Gerkau, Christine R. Rose & Nanna MacAulay (2022) Acetazolamide modulates intracranial pressure directly by its action on the cerebrospinal fluid secretion apparatus. Fluids and Barriers of the CNS 19:53
- Søren N. Andreassen, Trine L. Toft-Bertelsen, Jonathan H. Wardman, Rene Villadsen & Nanna MacAulay (2022) Transcriptional profiling of transport mechanisms and regulatory pathways in rat choroid plexus. Fluids and Barriers of the CNS 19:44
- Nanna MacAulay, Richard F. Keep & Thomas Zeuthen (2022) Cerebrospinal fluid production by the choroid plexus: a century of barrier research revisited. Fluids and Barriers of the CNS 19:26
- Nanna MacAulay (2021) Molecular mechanisms of brain water transport. Nature Reviews Neuroscience 6:326-344
- Annette B. Steffensen, Eva K. Oernbo, Anca Stoica, Niklas J. Gerkau, Dagne Barbuskaite, Katerina Tritsaris, Christine R. Rose & Nanna MacAulay (2018) Cotransporter-mediated water transport underlying cerebrospinal fluid formation. Nature Communications 9:2167
Born 15th December 1972
Education
2020 DMSci, Faculty of Health Sciences, UCPH
2002 PhD, Faculty of Health Sciences, UCPH
1998 MSc in biochemistry, McGill University, Canada
1995-96 Student exchange to McGill University, Canada
1995 BSc in biology, UCPH
Positions
2019- Professor at Department of Neuroscience UCPH
2015-2018 Associate professor at Department of Neuroscience UCPH
2006-2015 Associate professor at Department of Cellular and Molecular Medicine, UCPH
2004-2006 Associate professor at Department of Medical Physiology, UCPH
2002-2003 Postdoc at Department of Medical Physiology, UCPH
Prizes and Awards
2023 Lundbeck Foundation’s Scientific Enrichment Prize
2023 Fondsbørsvekselerer H Hansen og hustrus scholarship
2020 Nominated for DFF’s Elite Researcher (as one out of eight from UCPH)
2019 Leo Dannin’s Research prize
2012 DFF’s Starting Grant award ‘Sapere Aude’
2008 L’Oreal, Unesco and the Royal Danish Academy of Sciences’ award
2006 Danish Independent Research Council’s award ’Young Elite Researcher’
1996 Scholarship from Medical Research Council of Canada, McGill University
Funding ID (since 2011 only)
2024-2030 Lundbeck Foundation Professorship: 20 MDKK
2022-2025 Friis Foundation: 0.5 MDKK
2021-2024 Michaelsen’s Foundation: 0.5 MDKK
2020-2023 Independent Research Fund Denmark: 2.5 MDKK
2020-2024 Lundbeck Foundation Ascending investigator grant: 5 MDKK
2020-2021 Carlsberg Foundation: 0.3 MDKK
2019-2023 Lundbeck Foundation Thematic Grant (co-applicant, my share): 4.4 MDKK
2019-2021 NovoNordisk Foundation Symposium: 0.25 MDKK
2018-2022 IMK Almene Foundation: 0.9 MDKK
2018-2022 Friis’ Foundation: 0.5 MDKK
2018-2019 Brdr. Hartmann’s Foundation: 0.5 MDKK
2017-2022 NovoNordisk Foundation – Tandem (main applicant, my share): 7.5 MDKK
2019-2020 National Institute of Health (co-investigator, my share): 0.15 MDKK
2016-2018 NovoNordisk Foundation: 1.2 MDKK
2016-2019 Augustinus Foundation: 0.3 MDKK
2016-2017 Michaelsen’s Foundation: 0.3 MDKK
2016-2017 Carslberg Foundation: 0.1 MDKK
2014-2017 Lundbeck Foundation: 1.6 MDKK
2014-2015 Friis’ Foundation: 0.3 MDKK
2013-2016 Thorberg’s Foundation: 2.5 MDKK
2012-2017 Independent Research Fund Denmark (Sapere Aude): 6.8 MDKK
2011-2014 Independent Research Fund Denmark 1.8 MDKK
2011-2014 Lundbeck Foundation: 0.7 MDKK
Commissions of trust and Meeting organization
2025 - Vice Chair of Department for Research and Innovation
2023 – International Brain Barriers Society steering council member
2022 Co-organiser of Matchpoints – our fabulous brain, Aarhus University
2021 – 2022 Reviewer for the Portuguese Research Council
2021 BrainH2O Symposium, UCPH
2019 – Board member Jakobinerklubben
2019 – 2022 Panel member British Royal Society International Exchanges
2019 – Associate Editor, Fluids and Barriers in the CNS
(editorial board member 2016-)
2017 – 2020 Member of Department of Neuroscience management team
2016 – Head of the Graduate Program in Neuroscience (NeuroGrad), UCPH
2016 – Organizer of the Neuroseminar series, Department of Neuroscience
2016 BrainWater Minisymposium, UCPH
2016 Scientific board of ‘the 19th Int. Symposium on Signal Transd. at the BBBs’
2013 – Editorial Board Member, GLIA
2013 – 2016 Co-chair of the Society of Physiology’s special interest group
“Molecular physiology of channels and transporters’
2011 – 2016 Strategic research committee at the Faculty of Health, UCPH
2008 – 2015 Departmental research committee, Dept of Cell and Mol Med, UCPH
2008 – 2015 SPS ‘Scand. Conference on Mol. Physiol. of Channels and Transporters’, SE
In vivo animal experimentation on anesthetized rodents (determination of CSF production rate, intracranial pressure, CSF outflow resistance, brain water content, etc.)
Two-electrode voltage clamp and volume measurement of Xenopus oocytes (heterologously expressing transport proteins)
Radio-isotope flux measurements (in ex vivo tissue, cell culture, Xenopus oocytes)
Molecular biology (plasmid preparation, mutagenesis, subcloning, sequencing, in vitro RNA transcription, etc.)
Western blot, surface biotinylation, immunostaining, etc.
2025
120. Sara D. Lolansen, Eszter O. Révész, Søren N. Andreassen, Marleen Trapp, Chiara Salio, Marco Sassoé-Pognetto, Jens Velde Andersen, Emil W. Westi, Trine L. Toft-Bertelsen, Jonathan H. Wardman, Anne-Kristine Meinild Lundby, Flemming Dela, Annarita Patrizi, Blanca I. Aldana & Nanna MacAulay (2025) Choroid plexus-mediated CSF secretion remains stable in aging rats via high and age-resistant metabolic activity. Nature Communications accepted for Publication.
119. Kira Devantier, Trine L. Toft-Bertelsen, Andreas Prestel, Viktoria M.S. Kjær, Cagla Sahin, Marco Giulini, Stavroula Louka, Katja Spiess, Katrine Qvortup, Trond Ulven, Bo H. Bentzen, Alexandre M.J.J. Bonvin, Nanna MacAulay, Birthe B. Kragelund & Mette M. Rosenkilde (2025) The SH Protein of Mumps Virus is a Druggable Pentameric Viroporin. Science Advances sciadv.ads3071
118. Beatriche L. Edelbo, Annette B. Steffensen, Eszter O. Revesz, Søren N. Andreassen & Nanna MacAulay (2025) Modulation of cerebrospinal fluid secretion facilitated by serotonergic and noradrenergic receptors in the rat choroid plexus. Fluids and Barriers of the CNS 22:54
117. Brian S. Nielsen, Brian R. Larsen, Afnan B. Ghazal, Adriana Katz, KC Brennan, Steven J.D. Karlish & Nanna MacAulay (2025) Glial Versus Neuronal Na+/K+-ATPase in Activity-Evoked K+Clearance and Their Sensitivity to Elevated Extracellular K+. GLIA online ahead of print
116. Mette N. Jensen, Ida M.E. Israelsen, Jonathan H. Wardman, Dennis B. Jensen, Dan B. Andersen, Trine L. Toft-Bertelsen, Martin F. Rath, Jens J. Holst, Mette M. Rosenkilde & Nanna MacAulay (2025) Glucagon-like peptide-1 receptor modulates cerebrospinal fluid secretion and intracranial pressure in rats. Fluids and Barriers of the CNS 22:41
115. Trine L. Toft-Bertelsen, Beatriche L. Edelbo, Annette B. Steffensen, Sara D. Lolansen, Jonathan H. Wardman, Dennis B. Jensen & NannaMacAulay (2025) The cerebrospinal fluid secretion rate increases in awake and freely moving rats but differs with experimental methodology. Advanced Science, e2412469
114. Dennis B. Jensen, Trine L. Toft-Bertelsen, Dagne Barbuskaite, Jane Stubbe, Sandor Nietzsche, Tenna Capion, Nicolas H. Norager, Markus H. Olsen, Andreas T. Sørensen, Henrik Dimke, Christian A. Hübner, Marianne Juhler & Nanna MacAulay (2025) The Na+,K+,2Cl-cotransporter, not aquaporin 1, sustains cerebrospinal fluid secretion while controlling brain K+homeostasis. Advanced Science, e2409120
2024
113. Trine L. Toft-Bertelsen, Søren N. Andreassen, Nicolas H. Norager, Anja H. Simonsen, Steen G. Hasselbalch, Marianne Juhler & Nanna MacAulay (2024) Differential lipid signatures of lumbar and cisternal cerebrospinal fluid. Biomolecules. 14: 1431
112. Trine L. Toft-Bertelsen, Søren N. Andreassen, Anja H. Simonsen, Steen G. Hasselbalch & Nanna MacAulay (2024) The CSF lipid profile in patients with probable idiopathic normal pressure hydrocephalus differs from control but does not differ between shunt responders and non-responders. Brain Communications 6:fcae388
111. Sara D. Lolansen, Nina Rostgaard, Markus H. Olsen, Maud E. Ottenheijm, Lydia Drici, Tenna Capion, Nicolas H. Nørager, NannaMacAulay & Marianne Juhler (2024) Proteomic profile and predictive markers of outcome in patients with subarachnoid hemorrhage. Clinical Proteomics 21:51
110. Julia Castro-Arnau, Francois Chauvigne, Trine L. Toft-Bertelsen, Roderick N. Finn, Nanna MacAulay, Joan Cerda (2024) Aqp4a and Trpv4 mediate regulatory cell volume increase for swimming maintenance of marine fish spermatozoa. Cellular and Molecular Life Sciences 81:285
109. Inyoung Jeong, Søren N. Andreassen, L Hoang, M Poulain, Y Seo, HC Park, M Fürthauer, Nanna MacAulay, Nathalie Jurisch-Yaksi (2024) The evolutionary conserved choroid plexus contributes to the homeostasis of brain ventricles in zebrafish. Cell Reports 43(6):114331
108. Kristopher Kahle, Petra M. Klinge, Jenna E. Koschnitzky, Nanna MacAulay, Steven Robinson, SJ Schiff, Jennifer M. Strahle (2024) Pediatric hydrocephalus. Nature Reviews Disease Primers 10(1):35
107. Jonathan H. Wardman, Søren N Andreassen, Trine L. Toft-Bertelsen, Mette N Jensen, Jens E Wilhjelm, Bjarne Styrishave, Steffen Hamann, Steffen Heegaard, Alexandra J Sinclair A & NannaMacAulay (2024) CSF hyperdynamics in rats mimicking the obesity and androgen excess characteristic of patients with idiopathic intracranial hypertension. Fluids Barriers CNS 21(1):10
106. Michael Thormann, N Traube, N Yehia, R Koestler, G Galabova, NannaMacAulay, Trine L Toft-Bertelsen (2024) Toward New AQP4 Inhibitors: ORI-TRN-002. International Journal of Molecular Sciences 25(2):924
2023
105. Nina Rostgaard, Markus H. Olsen, Sara D. Lolansen, Nicolas H. Nørager, Peter Plomgaard, Nanna MacAulay & Marianne Juhler (2023) Ventricular CSF proteomic profiles and predictors of surgical treatment outcome in chronic hydrocephalus. Acta Neurochirurgica, 165(12):4059-4070
104. Nanna MacAulay & Trine L. Toft-Bertelsen (2023) Dual function of the choroid plexus: cerebrospinal fluid production and control of brain ion homeostasis. Cell Calcium, 116:102797
103. Trine L. Toft-Bertelsen, Søren N. Andreassen, Nina Rostgaard, Markus H. Olsen, Nicolas H. Nørager, Tenna Capion, Marianne Juhler & Nanna MacAulay (2023) Distinct cerebrospinal fluid lipid signature in patients with subarachnoid hemorrhage-induced hydrocephalus. Biomedicines 11:2360
102. Shai D. Ben-Shoshan, Sara D. Lolansen, Tiit I. Mathiesen & Nanna MacAulay (2023) CSF hypersecretion versus impaired CSF absorption in posthemorrhagic hydrocephalus: a systematic review. Acta Neurochirurgica, 165:3271-3287
101. Beatriche L. Edelbo, Søren N. Andreassen, Annette B. Steffensen & Nanna MacAulay (2023) Day-night fluctuations in choroid plexus transcriptomics and cerebrospinal fluid metabolomics. Proceedings of the National Academy of Sciences U.S.A. Nexus 2:pgad262
100. Connar S.J. Westgate, Christina Kamp-Jensen, Ida M. E. Israelsen, Trine Toft-Bertelsen, Jonathan H. Wardman, Christian A. Jensen, Bjarne Styrishave, Nanna MacAulay, Rigmor H. Jensen, Sajedeh Eftekhari (2023) Acetazolamide and topiramate lower intracranial pressure through different mechanisms: the effect of acute and chronic administration. British Journal of Pharmacology, online ahead of print
99. Sara D. Lolansen, Nina Rostgaard, Tenna Capion, Nicolas H. Nørager, Markus H. Olsen, Marianne Juhler, Tiit I. Mathiesen & Nanna MacAulay (2023) Posthemorrhagic hydrocephalus in patients with subarachnoid hemorrhage occurs independently of CSF osmolality. International Journal of Molecular Science 24:11476
98. Sara D. Lolansen, Dagne Barbuskaite, Fenghui Ye, Jianming Xiang, Richard Keep & Nanna MacAulay (2023) Spontaneously hypertensive rats can become hydrocephalic despite undisturbed secretion and drainage of cerebrospinal fluid. Fluids and Barriers of the CNS 20:53
97. Anette B. Steffensen, Beatriche L. Edelbo, Dagne Barbuskaite, Søren N. Andreassen, Markus H. Olsen, Kirsten Møller & Nanna MacAulay (2023) Nocturnal increase in cerebrospinal fluid secretion as a circadian regulator of intracranial pressure. Fluids and Barriers of the CNS 20:49
96. Jonathan H. Wardman, Mette Nyholm Jensen, Søren Norge Andreassen, Bjarne Styrishave, Jens E. Wilhjelm, Alexandra J. Sinclair & Nanna MacAulay (2023) Modelling idiopathic intracranial hypertension in rats: contributions of high fat diet and testosterone to intracranial pressure and cerebrospinal fluid production. Fluids and Barriers of the CNS 20:44
95. Nina Rostgaard, Markus H. Olsen, Tenna Capion, Nanna MacAulay & Marianne Juhler (2023) Inflammatory markers as predictors of shunt dependency and functional outcome in patients with aneurysmal subarachnoid hemorrhage. Biomedicines 11:997
94. Pooya R. Khamesi, V. Charitatos, Eva K. Heerfordt, Nanna MacAulay & Vartan Kurtcuoglu (2023) Are standing osmotic gradients the main driver of cerebrospinal fluid production? A computational analysis. Fluids and Barriers of the CNS 20:18
93. Nina Rostgaard, Markus H. Olsen, M. Ottenheijm, L. Drici, Anja H. Simonsen, Per Plomgaard, Hanne Gredal, Helle H. Poulsen, Henrik Zetterberg, Kaj Blennow, Steen G. Hasselbalch, Nanna MacAulay & Marianne Juhler (2023) Differential proteomic profile of lumbar and ventricular cerebrospinal fluid. Fluids and Barriers of the CNS 20:6
2022
92. Trine L Toft-Bertelsen, Dagne Barbuskaite, Eva K Heerfordt, Sara D Lolansen, Søren N Andreassen, Nina Rostgaard, Markus H Olsen, Nicolas H. Norager, Tenna Capion, Martin F Rath, Marianne Juhler & Nanna MacAulay (2022) Lysophosphatidic acid as a CSF lipid in posthemorrhagic hydrocephalus that drives CSF accumulation via TRPV4-induced hyperactivation of NKCC1. Fluids and Barriers of the CNS 19:69
91. Eva K. Oernbo, Annette B. Steffensen, Pooya R Khamesi, Trine L Toft-Bertelsen, Dagne Barbuskaite, Frederik Vilhardt, Niklas J. Gerkau, Katerina Tritsaris, Anja H Simonsen, Sara D Lolansen, Søren N Andreassen, Steen G. Hasselbalch, Thomas Zeuthen, Christine R. Rose, Vartan Kurtcuoglu & Nanna MacAulay (2022) Membrane transporters control cerebrospinal fluid formation independently of conventional osmosis to modulate intracranial pressure. Fluids and Barriers of the CNS 19:65
90. Sara D. Lolansen, Nina Rostgaard, Dagne Barbuskaite, Tenna Capion, Markus H. Olsen, Nicolas H. Norager, Frederik Vilhardt, Søren N. Andreassen, Trine L. Toft-Bertelsen, F. Ye, Marianne Juhler, Richard Keep & Nanna MacAulay (2022) Posthemorrhagic hydrocephalus associates with elevated inflammation and CSF hypersecretion via activation of choroidal transporters. Fluids and Barriers of the CNS 19:62
89. Dagne Barbuskaite, Eva K. Oernbo, Jonathan H. Wardman, Trine L. Toft-Bertelsen, Eller Conti, Søren N. Andreassen, Niklas J. Gerkau, Christine R. Rose & Nanna MacAulay (2022) Acetazolamide modulates intracranial pressure directly by its action on the cerebrospinal fluid secretion apparatus. Fluids and Barriers of the CNS 19:53
88. Eva K.Oernbo, Annette B. Steffensen, Hanne Gredal, Helle H. Poulsen, Nina Rostgaard, CH Rasmussen, M Møller-Nissen, Anja H. Simonsen, Steen G. Hasselbalch, Marianne Juhler & Nanna MacAulay (2022) Cerebrospinal fluid osmolality cannot predict development or surgical outcome of idiopathic normal pressure hydrocephalus. Fluids and Barriers of the CNS 19:52
87. Søren N. Andreassen, Trine L. Toft-Bertelsen, Jonathan H. Wardman, Rene Villadsen & Nanna MacAulay (2022) Transcriptional profiling of transport mechanisms and regulatory pathways in rat choroid plexus. Fluids and Barriers of the CNS 19:44
86. Marianne Juhler, TS Hansen, HVG Novrup, Nanna MacAulay & Tina N. Munch (2022) Hydrocephalus study design: testing new hypotheses in clinical studies and bench-to-bedside research. World Neurosurgery 161:424-431
85. Nanna MacAulay, Richard F. Keep & Thomas Zeuthen (2022) Cerebrospinal fluid production by the choroid plexus: a century of barrier research revisited. Fluids and Barriers of the CNS 19:26
2021
84. Sara Diana Lolansen, Nina Rostgaard,Søren Norge Andreassen, Anja Hviid Simonsen,Marianne Juhler, Steen Gregers Hasselbalch & Nanna MacAulay (2021) Elevated CSF inflammatory markers in patients with idiopathic normal pressure hydrocephalus do not promote NKCC1 hyperactivity in rat choroid plexus. Fluids and Barriers of CNS 18:54
83. Monica Lakk, GF Hoffmann, A. Gorusupudi, E Enyong, A Lin, PS Bernstein, Trine L. Toft-Bertelsen, Nanna MacAulay, MH Elliott, David Križaj (2021) Membrane cholesterol regulates TRPV4 function, cytoskeletal expression, and the cellular response to tension. Journal of Lipid Research 62:100145
82. Trine L. Toft-Bertelsen & Nanna MacAulay (2021) TRPing on cell swelling - TRPV4 senses it. Frontiers of Immunology 12:730982
81. Nanna MacAulay (2021) Reply to ‘Aquaporin 4 and glymphatic flow have central roles in brain fluid homeostasis’. Nature Reviews Neuroscience 10:651-652
80. Nanna MacAulay (2021) Molecular mechanisms of brain water transport. Nature Reviews Neuroscience 6:326-344
79. Sara D. Lolansen, Nina Rostgaard, Eva Kjer Oernbo, Marianne Juhler, Anja Hviid Simonsen & Nanna MacAulay (2021). Inflammatory markers in cerebrospinal fluid from patients with hydrocephalus: a systematic literature review. Disease Markers 2021:8834822
78. Trine L. Toft-Bertelsen & Nanna MacAulay (2021) TRPing to the point of clarity: understanding the function of the complex TRPV4 ion channel. Cells 10:165
2020
77. G.V. Mkrtchyan, K. Abdelmohsen, P. Andreux,... ...Nanna MacAulay... ... et al. (2020) ARDD 2020: from aging mechanisms to interventions. Aging 12:24484-24503
76. Nanna MacAulay & Christine C. Rose (2020) Rebuttal from Nanna MacAulay & Christine C. Rose. Journal of Physiology 598:4743
75. Nanna MacAulay & Christine C. Rose (2020) CrossTalk opposing view: NKCC1 in the luminal membrane of choroid plexus is outwardly directed under basal conditions and contributes directly to cerebrospinal fluid secretion. Journal of Physiology 598: 4737-4739
74. Trine Lisberg Toft-Bertelsen, Brian Roland Larsen, Sofie Kjellerup Christensen, Himanshu Khandelia, Helle S. Waagepetersen & Nanna MacAulay (2020) Clearance of activity-evoked K+ transients and associated glia cell swelling occur independently of AQP4; a study with an isoform-selective AQP4 inhibitor. GLIA 69: 28-41
73. Nanna MacAulay (2020) Molecular mechanisms of K+ clearance and extracellular space shrinkage-Glia cells as the stars. GLIA 68:2192-2211
72. Brian Skriver Nielsen, Trine Lisberg Toft-Bertelsen, Sara D. Lolansen, Connor L. Anderson, Morten Schak Nielsen, Roger J. Thompson & Nanna MacAulay (2020) Pannexin 1 activation and inhibition is permeant-selective. Journal of Physiology 598:361-379
2019
71. Trine Lisberg Toft-Bertelsen, Oleg Yarishkin, Sara Redmon,Tam TT Phuong, David Krizaj & Nanna MacAulay (2019) Volume sensing in the transient receptor potential vanilloid 4 ion channel is cell type-specific and mediated by an N-terminus volume-sensing domain. Journal of Biological Chemistry 294:18421-18434
70. Brian Skriver Nielsen, Francesco Zonta, Thomas Farkas, Thomas Litman, Morten Schak Nielsen & Nanna MacAulay (2019) Structural determinants underlying permeant discrimination of the Cx43 hemichannel. Journal of Biological Chemistry 294:16789-16803
69. Kasper Lykke, Mette Assentoft, Sofie Hørlyck, Hans Christian C. Helms, Anca Stoica, Trine L. Toft-Bertelsen, Katerina Tritsaris, Frederik Vilhardt, Birger Brodin & Nanna MacAulay (2019) Evaluating the involvement of cerebralmicrovascular endothelial Na+/K+-ATPase and Na+-K+-2Cl- co-transporter (NKCC1) in electrolyte fluxes in an in vitro BBB model of dehydration. Journal of Cerebral Blood Flow and Metabolism 39:497-512
68. Brian Roland Larsen, Anca Stoica, Nanna MacAulay (2019) Developmental maturation of activity-induced K+ and pH transients and the associated extracellular space dynamics in the rat hippocampus. Journal of Physiology 597:583-597
2018
67. Claudia Brandt, P Seja, Katrin Töllner, Kerstin Römermann, Philip Hampel, M Kalesse, A Kipper, Peter W Feit, Kasper Lykke, Trine L Toft-Bertelsen, P Paavilainen, I Spoljaric, Martin Puskarjov , Nanna MacAulay, Kai Kaila, Wolfgang Löscher (2018) Bumepamine, a brain-permeant benzylamine derivative of bumetanide, does not inhibit NKCC1 but is more potent to enhance phenobarbital's anti-seizure efficacy. Neuropharmacology 143:186-204
66. Eva Kjer Oernbo, Kasper Lykke, Annette B. Steffensen, Kathrin Töllner, Christina Kruuse, Martin Fredensborg Rath, Wolfgang Löscher & Nanna MacAulay (2018) Cerebral influx of Na+ and Cl- as the osmotherapy-mediated rebound response in rats. Fluids and Barriers of the CNS 15:27
65. Philip Hampel, Kerstin Römermann, Nanna MacAulay & Wolfgang Löscher (2018) Azosemide is more potent than bumetanide and various other loop diuretics to inhibit the sodium-potassium-chloride-cotransporter human variants hNKCC1A and hNKCC1B. Scientific Reports 8:9877
64. Annette B. Steffensen, Eva K. Oernbo,Anca Stoica, Niklas J. Gerkau, Dagne Barbuskaite, Katerina Tritsaris, Christine R. Rose & Nanna MacAulay (2018) Cotransporter-mediated water transport underlying cerebrospinal fluid formation. Nature Communications 9:2167
63. Katja Stahl, S. Rahmani, A. Prydz, N. Skauli, Nanna MacAulay, M.N. Mylonakou, R. Torp, Ø. Skare, T. Berg, T.B. Leergaard, R.E. Paulsen, O.P. Ottersen, Mahmood Amiry-Moghaddam (2018) Targeted deletion of the aquaglyceroporin AQP9 is protective in a mouse model of Parkinson's disease. PLoS One 13:e0194896
62. Trine L. Toft-Bertelsen, Brian Roland Larsen & NannaMacAulay (2018) Sensing and regulation of cell volume - we know so much and yet understand so little: TRPV4 as a sensor of volume changes but possibly without a volume-regulatory role? Channels 12;100-108
61. Siren Berland, Trine L. Toft-Bertelsen, Ingvild Aukrust, Jan Byska, Marc Vaudel, Laurence A. Bindoff, Nanna MacAulay (shared senior authorship) & Gunnar Houge (2018)A denovo Ser111Thr variant in aquaporin-4 in a patient with intellectual disability, transient signs of brain ischemia, transient cardiac hypertrophy, and progressive gait disturbance. Molecular Case Studies 4, pii: a002303
2017
60. Brian Skriver Nielsen, Jette Skov Alstrom,Bruce J. Nicholson, Morten Schak Nielsen & Nanna MacAulay (2017)Permeant-specific gating of connexin 30 hemichannels. Journal of Biological Chemistry 292:19999-20009
59. Lena Rosenbaek, Federica Rizzo, Qi Wu, Lorena Rojas-Vega, Gerardo Gamba Ayala, Nanna MacAulay, Oliver Staub & Robert A. Fenton (2017) The thiazide sensitive sodium chloride co-transporter NCC is modulated by site-specific ubiquitylation. Scientific Reports 7:12981
58. Anca Stoica, Brian Roland Larsen, Mette Assentoft, Rikke Holm, Leanne M. Holt, Frederik Vilhardt, Bente Vilsen, Karin Lykke-Hartmann, Michelle L. Olsen & Nanna MacAulay (2017) The α2β2 isoform combination dominates the astrocytic Na+/K+-ATPase activity and is rendered nonfunctional by the α2.G301R familial hemiplegic migraine type 2-associated mutation. GLIA 65:1777-1793
57. Brian Roland Larsen & Nanna MacAulay (2017) Activity-dependent astrocyte swelling is mediated by pH-regulating mechanisms. GLIA 65:1668-1681
56. Lena L. Rosenbaek. F. Rizzo, Nanna MacAulay, Olivier Staub & Robert A. Fenton (2017) Functional assessment of sodium chloride co-transporter NCC mutants in polarized mammalian epithelial cells. American Journal of Physiology - Renal Physiology 313:495-504
55. Brian s. Nielsen, Daniel B. Hansen, Bruce R. Ransom, Morten S. Nielsen & Nanna MacAulay (2017) Connexin hemichannels in astrocytes: an assessment of controversies regarding their functional characteristics. Neurochemical Research 42:2537-2550
54. Trine L. Toft-Bertelsen, David Krízaj & Nanna MacAulay (2017) When size matters: transient receptor potential vanilloid 4 channel as a volume-sensor rather than an osmo-sensor. Journal of Physiology 595:3287-3302
2016
53. Emma Olesen, Hanne Moeller, Mette Assentoft, Nanna MacAulay & Robert Fenton (2016) The vasopressin type-2 receptor and prostaglandin receptors EP2 and EP4 can increase aquaporin-2 plasma membrane targeting through a cAMP independent pathway. American Journal of Physiology – Renal Physiology 311:935-944
52. Mette Assentoft, Shreyas Kaptan, Hans-Peter Schneider, Joachim W. Deitmer, Bert L. de Groot & Nanna MacAulay (2016) Aquaporin 4 as a NH3channel. Journal of Biological Chemistry 291:19184-19195
51. P. Wanitchakool, J. Ousingsawat, L. Sirianant, Nanna MacAulay, Rainer Schreiber & Karl Kunzelmann (2016) Cl-channels in apoptosis. European Biophysical Journal 45: 599-610
50. Brian Roland Larsen, Rikke Holm, Bente Vilsen & Nanna MacAulay (2016) Glutamate transporter activity promotes enhanced Na+/K+-ATPase-mediated extracellular K+management during neuronal activity. Journal of Physiology 594:6627-6641
49. Brian Roland Larsen, Anca Stoica & Nanna MacAulay (2016) Managing brain extracellular K+ during neuronal activity: The physiological role of the Na+/K+-ATPase subunit isoforms. Frontiers of Physiology 7(141):fphys.2016.00141
48. Kasper Lykke, Kathrin Töllner, Peter W. Feit, Thomas Erker, Nanna MacAulay & Wolfgang Löscher (2016)The search for NKCC1-selective drugs: Structure-function relationship of bumetanide and various bumetanide derivatives in inhibiting the human cation-chloride cotransporter NKCC1A. Epilepsy & Behavior 59:42-49
47. Nils Landegren, M. Pourmousa Lindberg, J. Skov, Å. Hallgren, D. Eriksson, Trine Lisberg Toft-Bertelsen, Nanna MacAulay, E. Hagforsen, A. Räisänen-Sokolowski, H. Saha, T. Nilsson, G. Nordmark, S. Ohlsson, J. Gustafsson, E.S. Husebye, E. Larsson, M.S. Anderson, J. Perheentupa, F. Rorsman, Robert A. Fenton & O. Kämpe (2016) Autoantibodies targeting a collecting duct-specific water channel in tubulointerstitial nephritis. Journal of American Society of Nephrology 27:3220-3228
46. Hanne B. Moeller, J. Slengerik-Hansen, T. Aroankins, Mette Assentoft, Nanna MacAulay, Søren K. Moestrup, V. Bhalla & Fenton RA (2016) Regulation of the water channel aquaporin-2 via 14-3-3θ and -ζ. Journal of Biological Chemistry 291:2469-84
2015
45. Andrew Jo, Daniel A. Ryskamp, Tam T.T. Phuong, Alan S. Verkman, Oleg Yarishkin, Nanna MacAulay & David Križaj (2015). TRPV4 and AQP4 channels synergistically regulate cell volume and calcium homeostasis in retinal Müller glia. Journal of Neuroscience 35:13525-13537
44. Shreyas Kaptan, Mette Assentoft, Hans Peter Schneider, Robert A. Fenton, Joachim Deitmer, Nanna MacAulay & Bert de Groot (2015) Is H95 a pH-dependent gate in aquaporin 4? Structure 23:2309-2318
43. Jette S. Alstrom, Daniel B. Hansen, Morten S. Nielsen & Nanna MacAulay (2015) Isoform-specific phosphorylation-dependent regulation of connexin hemichannels. Journal of Neurophysiology 14:3014-22
42. Annette B. Steffensen, Jeremy Sword, Deborah Croom, Sergei A. Kirov & Nanna MacAulay (2015) Cotransporters as a molecular mechanism underlying spreading depolarization-induced dendritic beading. Journal of Neuroscience 35:12172-12187
41. Kasper Lykke, Mette Assentoft, Robert A. Fenton, Mette M. Rosenkilde & Nanna MacAulay (2015). Vasopressin receptors V1aand V2 are not osmo-sensors. Physiological Reports 3:phy2.12519
40. Kasper Lykke, Kathrin Töllner, Kerstin Römermann, Peter W. Feit, Thomas Erker, Nanna MacAulay & Wolfgang Löscher (2015) Structure-activity relationships of bumetanide derivatives: correlation between diuretic activity in dogs and inhibition of human NKCC2 variant A.British Journal of Pharmacology 172:4469-4480
39. Mette Assentoft, Brian Roland Larsen & Nanna MacAulay (2015) Regulation and function of AQP4 in the central nervous system.Neurochemical Research 40:2615-2627
38. Martin N. Andersen, Lasse Skibsbye, C. Tang, F. Petersen, Nanna MacAulay, Hanne B. Rasmussen & Thomas Jespersen (2015) PKC and AMPK regulation of Kv1.5 potassium channels. Channels 9:121-128
37. Jette S. Alstrom, Line W. Stroemlund, Morten S. Nielsen & Nanna MacAulay (2015) Protein kinase C-dependent regulation of connexin43 gap junctions and hemichannels. Biochemical Society Transaction 43:519-523
36. Daniel Ryskamp, Andrew Jo, Amber Frye, Felix Vazquez-Chona, Nanna MacAulay, Wallace Thoreson & David Krizaj (2015) Swelling and eicosanoid metabolites differentially gate TRPV4 channels in retinal neurons and glia. Journal of Neuroscience 34:15689-15700
2014
35. Brian Roland Larsen & Nanna MacAulay (2014) Kir4.1-mediated spatial buffering of K+: Experimental challenges in determination of its temporal and quantitative contribution to K+clearance in the brain. Channels 8:544-560
34. Mette Assentoft, Brian Roland Larsen, Emma T.B. Olesen, Robert A. Fenton & Nanna MacAulay (2014) AQP4 plasma membrane trafficking or channel gating is not significantly modulated by phosphorylation at C-terminal serine residues. American Journal of Physiology – Cell Physiology 307:957-965
33. Daniel Bloch Hansen, Ye Zu-Cheng, Kirstine Calloe, Thomas H. Braunstein, Johannes P. Hofgaard, Bruce R. Ransom, Morten S. Nielsen & Nanna MacAulay (2014) Activation, permeability, and inhibition of astrocytic and neuronal large pore (hemi)channels. Journal of Biological Chemistry 289:26058-26073
32. Daniel Bloch Hansen, Thomas H. Braunstein, Morten S. Nielsen & Nanna MacAulay (2014) Distinct permeation profiles of the connexin 30 and 43 hemichannels. FEBS Letters588:1446-1457
31. Brian Roland Larsen, Mette Assentoft, Maria L. Cotrina, Susan Z. Hua, Maiken Nedergaard, Kai Kaila, Juha Voipio & Nanna MacAulay (2014)Contributions of the Na+/K+-ATPase, NKCC1, and Kir4.1 to hippocampal K+-clearance and volume responses. GLIA 62:608-622
2013
30. Thomas Zeuthen, Magnus Alsterfjord, Eric Beitz & Nanna MacAulay (2013) Osmotic water transport in aquaporins. Evidence for a stochastic mechanism. Journal of Physiology 591:5017-5029
29. Rikke Sogaard, Lars Borre, Thomas H. Braunstein, Kenneth L. Madsen & Nanna MacAulay (2013) Functional modulation of the glutamate transporter variant GLT1b by the PDZ domain protein PICK1. Journal of Biological Chemistry 288:20195-20207
28. Mette Assentoft, Shreyas Kaptan, Robert A. Fenton, Susan Z. Hua, Bert L. de Groot & Nanna MacAulay (2013) Phosphorylation of rat aquaporin-4 at Ser111 is not required for channel gating. GLIA 61:1101-1112
2012
27. Lene L. Rosenbaek, Mette Assentoft, Nis B. Pedersen, Nanna MacAulay & Robert A. Fenton (2012) Characterization of a novel phosphorylation site in the sodium chloride transporter, NCC. Journal of Physiology 590:6121-6139
26. Nanna MacAulay & Thomas Zeuthen (2012) Glial K+ clearance and cell swelling: key roles for cotransporters and pumps. Neurochemical Research 37:2299-2309
25. Thomas Zeuthen & Nanna MacAulay (2012) Transport of water against its concentration gradient: fact or fiction? WIREs: Membrane Transport and Signaling 1(4):373-381 (doi:10.1002/wmts:54)
24. Thomas Zeuthen & Nanna MacAulay (2012) Cotransport of water by the Na+-K+-2Cl-cotransporter expressed in Xenopusoocytes: NKCC1 versus NKCC2. Journal of Physiology 590:1139-1154
23. Rikke Søgaard, Ivana Novak & Nanna MacAulay (2012) Differential effect of ammonia on three glutamate transporter isoforms expressed in Xenopusoocytes. American Journal of Physiology – Cell Physiology 302:880-891
2010
22. Robert A. Fenton, Hanne B. Moeller, Marina Zelenina, Marteinn T. Snaebjornsson, Torgeir Holen & Nanna MacAulay (2010) Differential water permeability and regulation of three AQP4 isoforms. Cellular and Molecular Life Sciences 67:829-840
21. Nanna MacAulay & Thomas Zeuthen (2010) Water transport between CNS compartments: contribution of aquaporins versus co-transporters. Neuroscience 168:941-956
2009
20. Hanne B. Moeller, Robert A. Fenton, Thomas Zeuthen & Nanna MacAulay (2009) Vasopressin-dependent short-term regulation of AQP4 expressed in Xenopusoocytes. Neuroscience 164:1674-1684
19. Nanna MacAulay, Steffen Hamann & Thomas Zeuthen (2009) Chloride transporters as water pumps: elements in a new model of epithelial water transport. In: Physiology and pathology of chloride transporters and channels in the nervous system. Eds FJ Alvarez-Leefmans & E Delpire. Academic Press, Elsevier pp 547-568
18. Rikke Soe, Nanna MacAulay & Dan Klaerke (2009) Modulation of Kir4.1 and Kir4.1-Kir5.1 channels by small changes in cell volume. Neuroscience Letters 457:80-84
17. Anne-Kristine Meinild, Don D. Loo, Søren Skovstrup, Ulrik Gether & Nanna MacAulay (2009) Elucidating conformational changes in the γ-aminobutyric acid (GABA)-transporter-1. Journal of Biological Chemistry 284:16226-16235
16. Rikke Søgaard, Magnus Alsterfjord, Nanna MacAulay & Thomas Zeuthen (2009) Ammonium ion transport by the AMT/Rh homolog TaAMT1;1 is stimulated by acidic pH. Pflugers Archives 458:733-743
15. Hanne B Moeller, Nanna MacAulay (shared first author), Mark A Knepper & Robert A Fenton (2009) Role of multiple phosphorylation sites in the carboxyl-terminal tail of aquaporin-2 for water transport: Evidence against channel gating. American Journal of Physiology - Renal Physiology 296:649-657
2007
14. Thomas Zeuthen, Emil Zeuthen & Nanna MacAulay (2007) Water transport by GLUT2 expressed in Xenopus laevis oocytes. Journal of Physiology579:345-361
2005
13. Aidas Alaburda, Raul Russo, Nanna MacAulay & Jørn Hounsgaard (2005) Periodic high and low conductance states in spinal motoneurons and interneurons during scratch-like network activity in adult turtles. Journal of Neuroscience 25:6316-6321
2004
12. Nanna MacAulay, Steffen Hamann & Thomas Zeuthen (2004) Water transport in the brain: role of cotransporters.Neuroscience 129:1031-1044
2003
11. Nanna MacAulay, Anne-Kristine Meinild, Thomas Zeuthen & Ulrik Gether (2003) Residues in the extracellular loop 4 are critical for maintaining the conformational equilibrium of the γ-aminobutyric acid (GABA) transporter-1. Journal of Biological Chemistry 278:28771-28777
10. Morten Grunnet, Thomas Jespersen, Nanna MacAulay, Nanna K. Jørgensen, Nicole Schmitt, Olaf Pongs, Søren-Peter Olesen & Dan Klærke (2003) KCNQ1 channels sense small changes in cell volume.Journal of Physiology 549:419-427
2002
9. Nanna MacAulay, Thomas Zeuthen & Ulrik Gether (2002) Conformational basis for the Li+-induced leak current in the rat γ-aminobutyric acid (GABA) transporter-1. Journal of Physiology 544:447-458
8. Nanna MacAulay, Ulrik Gether, Dan Klærke & Thomas Zeuthen (2002) Passive water and urea permeability of a human Na+/glutamate cotransporter expressed in Xenopus oocytes. Journal of Physiology 542:817-828
7. Morten Grunnet, Nanna MacAulay, Nanna K. Jørgensen, Bo S. Jensen, Søren-Peter Olesen & Dan Klærke (2002) Regulation of cloned Ca2+-activated K+channels by cell volume changes. Pflugers Archives 444:167-177
6. Thomas Zeuthen & Nanna MacAulay (2002) Passive water transport in biological pores. In International Review of Cytology, vol 215, eds T. Zeuthen & W.D. Stein., pp 259-284. Academic Press, San Diego.
5. Thomas Zeuthen & Nanna MacAulay (2002) Cotransporters as molecular water pumps. In International Review of Cytology,vol 215, eds T. Zeuthen & W.D. Stein., pp 203-230. Academic Press, San Diego.
2001
4. Nanna MacAulay, Annie Bendahan, Claus Juul Loland, Thomas Zeuthen, Baruch I. Kanner & Ulrik Gether (2001) Engineered Zn2+ switches in the GABA transporter-1: differential effects on GABA uptake and currents. Journal of Biological Chemistry276:40476-40485
3. Nanna MacAulay, Ulrik Gether, Dan Klærke & Thomas Zeuthen (2001) Water transport by the Na+-coupled glutamate cotransporter expressed in Xenopusoocytes. Journal of Physiology 530:367-378
1998
2. Rhoda Blostein, Stewart E. Daly, Nanna Boxenbaum, Lois K. Lane, José M. Arguello, Jerry B. Lingrel, Steve J. D. Karlish & Michael J. Caplan (1998) Conformational alterations resulting from mutations in cytoplasmic domains of the α-subunit of the Na,K-ATPase. Acta Physiologica Scandinavia (Supplement) 643:275-281
1. Nanna Boxenbaum, Stewart E. Daly, Zahid Z. Javaid, Lois K. Lane & Rhoda Blostein (1998) Changes in steady-state conformational equilibrium resulting from cytoplasmic mutations of the Na,K-ATPase α-subunit. Journal of Biological Chemistry 273:23086-23092
Lab members
| Name | Title | Job responsibilities | |
|---|---|---|---|
| Search in Name | Search in Title | Search in Job responsibilities | |
| Cecilie Retzlaff Hvass | PhD Fellow | MacAulay Lab |
|
| Ida Marchen Egerod Israelsen | Postdoc | MacAulay Lab |
|
| Nanna MacAulay | Professor | MacAulay Lab |
|
| Thomas Zeuthen | Professor Emeritus | MacAulay Lab |
|
| Thomas Rømhild Høj Nielsen | PhD Fellow | MacAulay Lab |
|
| Trine Lisberg Toft | Associate Professor | MacAulay lab |
|

