Kornum Lab
Our lab explores sleep biology in health and disease. Our focus is to understand sleep-wake neurophysiology and how the immune system affects the sleep regulating networks of the brain. We are particularly interested in the hypocretin/orexin neurons, their signaling, and their involvement in the sleep disorder narcolepsy and in sleep-wake regulation during sickness in general.
The Kornum Lab studies sleep-wake neurophysiology with a focus on the hypocretin (hcrt, also called orexin) system. We study how sleep is affected by the immune system, leading to disturbed sleep during sickness or even narcolepsy. We leverage single-cell technology, animal disease models and human genetics to explore and discover how hcrt neurons and surrounding cells, networks and pathways are involved in sleep disorder mechanisms.

Sleep is regulated by a complex network of brain areas and by many different neurotransmitters and other signalling compounds, including the hypocretin-producing neurons in the lateral hypothalamus.
Social interaction, play, and voluntary exercise have been shown to cause hypocretin activation. Some studies have also suggested that the hypocretin neurons are regulated by light. The implications of this are still unclear, but it looks like we here have a system that – when activated – helps us be really awake when needed.
The sleep disorder narcolepsy type 1 (NT1) is caused by the lack of hcrt, and for this reason NT1 is a focus area in the Kornum Lab. NT1 is caused by the patients losing their hypocretin producing neurons in hypothalamus likely due to an autoimmune pathogenesis. Both genetic and environmental predisposing factors point in this direction. We aim to discover what causes narcolepsy, and have over the years studied several aspects of the disease including neurophysiological, genetic, and neuroimmunological features.
Our findings in combination with those of many other research groups have confirmed the autoimmune hypothesis of NT1, but it has also led to many more questions. Autoreactive T-cells linked to NT1 are consistently found also in some healthy individuals (. For unknown reasons these individuals do not develop disease but are healthy long into adulthood past the normal onset age of NT1. The disease is also very heterogeneous. For example, around 25% develop co-morbid depression. It is unknown why. To better understand disease susceptibility and heterogeneity, it is important to study the brain and how environmental and behavioral factors influence the neuronal networks of the hypothalamus.
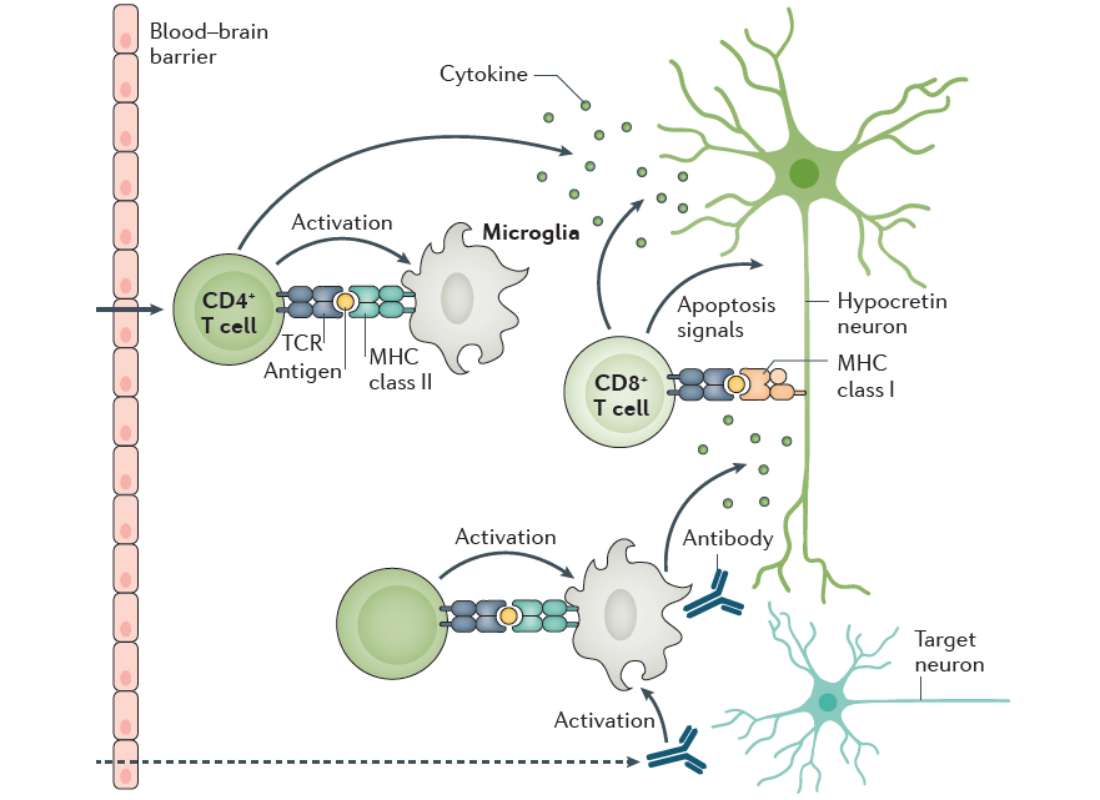
In our quest to understand what causes narcolepsy, we study the immune system of patients. We hypothesise that some of the proteins genetically associated with narcolepsy plays a central role in the disease process, and this is what we study. Importantly our work has shown that defects in the purinergic receptor P2Y11 predisposes to narcolepsy. P2Y11 is a dual Gs-Gq-protein coupled purinergic receptor that is activated by high concentrations of extracellular ATP. The receptor is highly important for the normal function of immune cells, and we are now studying how the narcolepsy associated mutations affects the immune system.
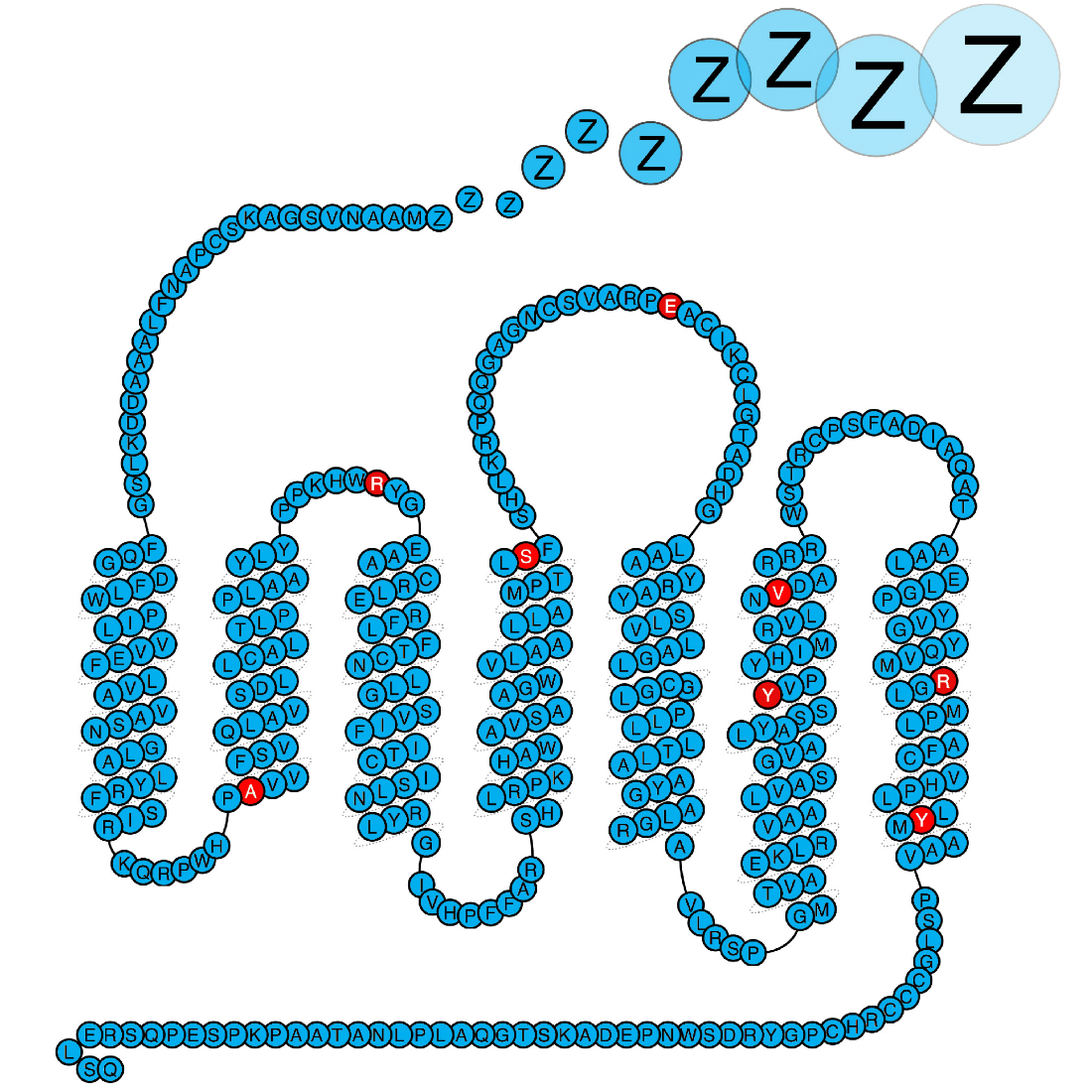
Kornum et al. Nature Genetics 2011, Degn et al. Brain 2017, Dreisig et al. Front. Immunol. 2018.
Se Birgittes profile page: https://researchprofiles.ku.dk/en/persons/birgitte-rahbek-kornum
- Animal models of sleep disorders: Transgenic models, in-house breeding, transgene expression using viral vectors and Cre-loxP-system.
- Sleep-wake electrophysiology in mice: We record 24-hour sleep-wake eeg/emg in behaving animals.
- Behavioural assessments: Locomotion. Spontaneous behaviour (grooming, eating, etc.)
- Single-cell RNA sequencing: We use droplet-based technologies to perform single-cell sequencing on cells and nuclei from brain tissue.
- General wet-lab techniques: Immunohistochemistry, quantitative RT-PCR, western blotting, etc.
- Machine learning: Deep Learning models for sleep data analysis
Lab members
| Name | Title | Job responsibilities | |
|---|---|---|---|
| Search in Name | Search in Title | Search in Job responsibilities | |
| Alberte Wollesen Jürgensen-Breum | PhD Fellow | Kornum Lab |
|
| Birgitte Rahbek Kornum | Professor | Kornum Lab |
|
| Javier Garcia Ciudad | PhD Fellow | Kornum Lab |
|
| Mie Gunni Kolmos | Master-Student | Kornum Lab |
|
| Nane Griem-Krey | Guest Researcher | Kornum Lab |
|
| Peter Kusk Jørgensen | Guest Researcher | Kornum Lab |
|
| Pomme Rigter | Postdoc | Kornum Lab |
|
| René Lemcke | Guest Researcher | Kornum Lab |
|

