Kiehn Lab
We study the molecular, cellular, and network diversification of locomotor circuitries in mammals with the goal of providing a unified understanding of the functional organization of neuronal circuits that execute movements.
A monumental challenge in neuroscience is to understand the operation of neuronal networks that are linked to execution of specific behaviors. Our lab is meeting this challenge by addressing the organization of neuronal networks that produce movements, the origin of all behaviors.
We study the molecular, cellular, and network diversification of locomotor circuitries in mammals with the goal of providing a unified understanding of the functional organization of neuronal circuits that execute movements. To this end, we apply new physiological and molecular genetic approaches, including optogenetics, RNA-seq, molecular tracing, advanced imaging, and electrophysiology.
We have deciphered the functional organization of spinal circuitries necessary for producing changes in timing and coordination of locomotion, and delineated brainstem circuits involved in gating or context-dependent selection of motor behaviors.
The lab also investigates plasticity in spinal networks and motor neurons following lesions of the spinal cord, with the goal of devising manipulations that may alleviate motor dysfunction following spinal cord injury.
In recent efforts, we also address the role of spinal interneurons in development and progression of amyotrophic lateral sclerosis.
Our work bridges the gap between neuronal circuit organization and behaviour, and has strong translational potential in development of therapies for movement disorders caused by trauma or disease.
Physiological and molecular organization of neuronal networks controlling movements in mammals
Movement takes many forms. Among movements, locomotion is one of the most fundamental—used by all animals and humans for interaction with the surroundings. Locomotion is employed episodically in many daily activities, representing an output measure for integrated brain activity involved in exploring the environment, escaping predators, and searching for food. Its activity also directly influences the state of sensory information processing.
The planning and initiation of locomotion takes place in the brain and brainstem, while the execution—which involves precise timing and coordination—is to a large part accomplished by activity within neuronal networks of the spinal cord itself.
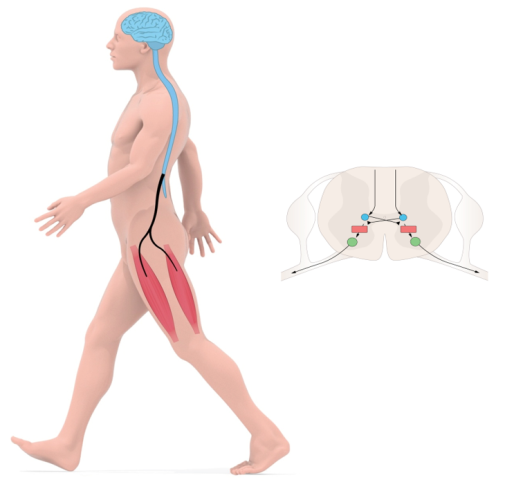
Early work from our lab has revealed aspects of the overall organization of spinal locomotor networks, the implication of cellular properties for rhythmicity, and the nature of neuronal spike coding.
Recent work has focused on the functional organization of key neuronal elements that characterize limbed locomotion in mammals: 1) rhythm generation, 2) coordination of flexors and extensors across the same or different joints in a limb or between limbs, and 3) left/right coordination.
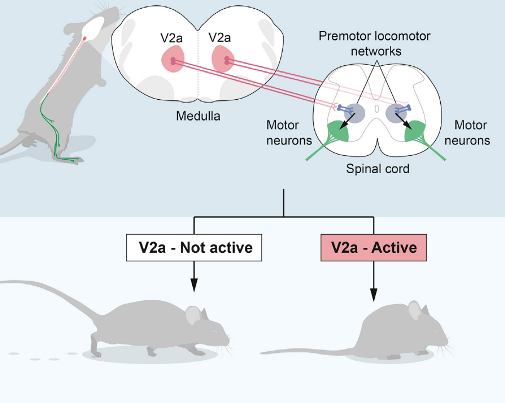
Rhythm generating neurons set tempo within the network, and are an elementary component of all vertebrate locomotor networks. Experiments from our lab using genetically driven expression of light-sensitive channels in excitatory and inhibitory neurons have demonstrated that excitatory neurons in the mammalian spinal cord are both sufficient and necessary for initiation and maintenance of rhythmic locomotor pattern. Using intersectional mouse genetics in combination with electrophysiology, we have identified non-overlapping subpopulations of excitatory neurons in the spinal cord that participate in rhythm generation.
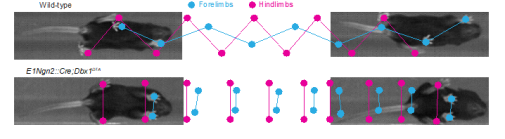
We have also characterized networks involved in left-right coordination, which include commissural interneurons (CINs) whose axons cross the midline, in detail using anatomical, electrophysiological, and targeted genetic ablation techniques for molecularly defined subpopulations of CINs.
These studies have converged on a common organizational principle for circuits controlling left-right alternation in mammals, which consists of a modular organization for left-right alternating gaits (walk and trot) and synchronous gaits (gallop/bound). Moreover, we have addressed the organization of circuits controlling flexor-extensor coordination. We are now addressing the functional integration of these diverse circuit elements using both in vitro and in vivo studies.
In a general scheme of motor control, we study how spinal locomotor circuits are activated and controlled by descending command signals. Decision-making signals to locomote are conveyed from the brain to locomotor regions in the midbrain. The locomotor regions in the midbrain include the mesencephalic locomotor region (MLR)—a complex structure—that in turn is thought to activate neurons in the reticular formation (RF) in the lower brainstem, which project to locomotor networks in the spinal cord. In the first optogenetics experiments in the mammalian locomotor system, we provided direct evidence that glutamatergic neurons in the lower brainstem can provide a ‘go’- signal sufficient to activate spinal locomotor networks.
We have now implemented in vivo optogenetic and chemogenetic approaches to probe the involvement of locomotor promoting and locomotor arresting areas in the brainstem, and further explore how these brainstem circuits are selected by upstream circuitries. These experiments have defined ‘start’ neurons in the midbrain, confined to the cuneiform nucleus and pedunculopontine nucleus, which cooperate to set locomotor speed and context-dependent selection of locomotor gate.
We have also defined ‘stop’ neurons in nucleus gigantocellularis that arrest ongoing locomotion.
In future studies we will use Parkinson disease models to probe the role of different motor structures in development and encoding of disease-induced gait disturbances.
Cellular mechanism underlying spasticity after spinal cord injury
Severe muscle spasticity develops as a consequence of spinal cord injury or damage to motor pathways from the brain. We previously found evidence that the pathophysiology of spasticity after spinal cord injury is related to chronic expression of plateau properties in motor neurons. Plateau potentials in vertebrate motor neurons are caused by activation of prolonged sodium/calcium currents, and their expression is dependent on activation of noradrenergic and/or serotoninergic receptors. The normal function of motor neuron plateau potentials seems to be to maintain persistent motor output and amplify synaptic inputs during rhythmic motor activity.
To find possible targets for regulation of plateau potentials after spinal cord injury, we have performed global gene expression profiling from rat motor neurons isolated before or after injury to the cord. These studies have shown that the chronic expression of plateaux may be related to changes in genes coding for the regulatory units for persistent sodium and calcium channels. In ongoing experiments, we are investigating how changes in interneuronal network activity interact with plateaux to generate spasticity using mouse genetic, calcium imaging, and in vivo optogenetic experiments. Our long-term goal is to define new therapies for symptoms associated with spinal cord injury.
- Hsu, Li-Ju, Bertho, Maelle Claire Valentine & Kiehn, Ole, 2023. Deconstructing the modular organization and real-time dynamics of mammalian spinal locomotor networks. Nature Communications. 14, 1, 18 p., 873. Contribution to journal › Journal article › Research › peer-review
- Goñi Erro, Haizea, Selvan, Raghav, Caggiano, V., Leiras, Roberto & Kiehn, Ole, 2023. Pedunculopontine Chx10 + neurons control global motor arrest in mice. Nature Neuroscience. 26, p. 1516–1528 43 p. Contribution to journal › Journal article › Research › peer-review
- Masini D, Kiehn O. Nat Commun (2022) Jan 26. Targeted activation of midbrain neurons restores locomotor function in mouse models of parkinsonism. Nature Communications. 2022 Jan 26;13(1):504. doi: 10.1038/s41467-022-28075-4.>PMID:35082287
- Leiras R, Cregg JM, Kiehn O. Annu Rev Neurosci. 2022 Jan 5. Brainstem Circuits for Locomotion. Annual Review of Neuroscience. 2022 Jan 5. doi: 10.1146/annurev-neuro-082321-025137. Online ahead of print.>PMID: 34985919
- Allodi I, Montañana-Rosell R, Selvan R, Löw P, Kiehn O (2021). Locomotor deficits in a mouse model of ALS are paralleled by loss of V1-interneuron connections onto fast motor neurons. Nature Communications. 12, Article number: 3251 (2021) >NatureComm
- Cregg M J, Leiras R, Montalant A, Wanken P, Wickersham R I, Kiehn O (2020). Brainstem neurons that command mammalian locomotor asymmetries. Nature Neuroscience. May 11. DOI: s41593-020-0633-7.>NatureNeuro
- Marcantoni M, Fuchs A, Löw P, Bartsch D, Kiehn O, Bellardita C (2020). Early delivery and prolonged treatment with nimodipine prevents the development of spasticity after spinal cord injury in mice. Science Translational Medicine. Apr 15. DOI: 10.1126/scitranslmed.aay0167.>Scitranslmed
- Caggiano V, Leiras R, Goñi-Erro H, Masini D, Bellardita C, Bouvier J, Caldeira V, Fisone G, Kiehn O (2018). Midbrain circuits that set locomotor speed and gait selection. Nature Jan 17. doi: 10.1038/nature25448. >http://rdcu.be/EV9n
- Kiehn O (2016). Decoding the organization of spinal circuits that control locomotion. Nat Rev Neurosci,17(4): 224-238.>pubmed
- Bouvier J, Caggiano V, Leiras R, Caldeira V, Bellardita C, Balueva K, Fuchs A, Kiehn O (2015). Descending Command Neurons in the Brainstem that Halt Locomotion. Cell,163(5): 1191-1203.>pubmed
- Dougherty KJ, Zagoraiou Z, Satoh D, Rozani I, Doobar S, Arber S, Jessell TM, Kiehn O. (2013). Locomotor Rhythm Generation Linked to the Output of Spinal Shox2 Excitatory Interneurons.Neuron,80(4): 920-933.>pubmed
- Talpalar AE, Bouvier J, Borgius L, Fortin G, Pierani A, Kiehn O (2013). Dual-mode operation of neuronal networks involved in left–right alternation. Nature, 500: 85–88.>pubmed
- Talpalar AE, Endo T, Löw P, Borgius L, Hägglund M, Dougherty KJ, Rygg J, Hnasko TS, Kiehn O (2011). Identification of minimal neuronal networks involved in flexor-extensor alternation in the mammalian spinal cord. Neuron, 71(6):1071-84.>pubmed
- Hägglund M, Borgius L, Dougherty KJ, Kiehn O. (2010). Activation of glutamatergic neurons in the mammalian brainstem or spinal cord evoke hind-limp locomotion. Nature Neuroscience, 13(2): 246-52.>pubmed
Born: September 30 1958. Citizenship: Danish/Swedish.
A. Education
2002: Docent, Karolinska Institutet, Sweden
1990: Doctor of Science from University of Copenhagen, Denmark
1985: M.D. from University of Copenhagen, Denmark
B. Current Position
2017-: Professor in Integrative Neuroscience, Department of Neuroscience, University of Copenhagen, Denmark.
2004-: Professor in Neuroscience, Department of Neuroscience, Karolinska Insitute (KI), Sweden.
C. Previous positions
2001-2004: Group-leader - via international ‘Elite-recruitment’ program at Karolinska Institute, Sweden.
1997-2001: Associate Professor, Department of Physiology, Copenhagen.
1995-2000: Hallas Møller Research Fellow Department of Physiology, Copenhagen.
1991-1995: Group Leader Institute of Neurophysiology, Copenhagen.
1989-1990: Postdoc, Sect. of Neurobiol. and Behavior Cornell University, USA.
1988-1989: Senior Research Associate, Institute of Neurophysiology, Copenhagen.
1985-1988: Junior Research Associate; Institute of Neurophysiology, Copenhagen.
1983-1985: Research Assistant Institute of Neurophysiology, Copenhagen.
D. Awards and honors
2024-30: Novo Nordisk Foundation Laureate continuation program
2022: Recipient of ‘The Brain Prize’ shared with Silvia Arber and Martyn Goulding.
2021: Recipient of Kirsten and Freddy Johansens Preclinical Research Prize
2019-: Lundbeck Foundation’s Professorship award.
2017-: Novo Nordic Foundation Laureate program.
2016-: European Research Council advanced grant (second award).
2014: Member of EMBO.
2013: Member of Academia Europea.
2012: Member of the Royal Swedish Academy of Sciences.
2010: Member of the The Royal Danish Academy of Sciences and Letters.
2011-2016: Research Professorship: Torsten and Ragnar Söderberg’s Professorship from the Royal Swedish Academy of Science.
2011-2016: European Research Council advanced grant.
2010-2015: 5 Year Distinguished Professor Award Karolinska Institute.
2004: Recipient of international Schellenberg Prize in spinal cord research.
2001-2006: 5 year recruitment via ‘Elite-recruitment’ program at Karolinska Institute.
1999-2001: PI for Human Frontier Science Program grant.
2001-2010: PI on R01 NIH grant to Kiehn Lab.
1995: Recipient of Hallas Møller Research Fellowship (5 year salary award from Novo Foundation – one/year in biomedicine).
1990: Recipient of Weimann Research Fellowship (5 year salary award).
E. Commissions of trust
2023-: Member of Brain Prize Election Committee.
2022-2024: President Elect for Federation of European Neuroscience Societies (FENS) (all European countries).
2024-2026: President FENS.
2022- Chair for Kirsten and Freddy Johansen’s Preclinical Research Prize Committee.
2019: Member of the Program Committee for the International Brain Organization Meeting.
2019: Co-editor in chief of Current Opinion in Neurobiology.
2017: Member of Steering Committee Department of Neuroscience UCPH.
2017: SAB Dandrite.
2016: Vice Chair Nobel Committee for Physiology or Medicine.
2014-2019: Elected member of the Nobel Committee for Physiology or Medicine.
2011-2013: Affiliated member of the Nobel Committee for Physiology or Medicine.
2008-2028: Elected Member of Nobel Assembly at Karolinska Institute.
2013: Evaluation panel consolidator ERC grant.
2012: Evaluation panel Söderberg’s foundation.
2010: Member of the Scientific Evaluation Committee for ETN in Zurich.
2008 & 2014: Scientific Evaluation Committee for Molecular Physiology of the Brain, Göttingen, Germany.
2006-2010: Member of the steering board of Stockholm Brain Institute.
2011-2015, 2015-: Co-director for StratNeuro at Karolinska Institute.
2003-2011: Deputy Chair at the Department of Neuroscience, Karolinska Institute.
2011-2012: Chair of Program Committee for Federation of European Neuroscience Societies (FENS).
2011-2013: Member of Executive committee for FENS.
2007-2009: Member of program committee for Society for Neuroscience USA.
2009: Member of Program Committee for FENS.
F. Leadership role
2021-: Vicechair, research – Department of Neuroscience, UCPH.
2020-23: Member of Rector’s Research Panel (6 members), UCPH.
2017–: Part of the steering group and founding member of the Department of Neuroscience, University of Copenhagen.
2006–2010: Member of the steering board of Stockholm Brain Institute.
2011–2016: Founding member and Co-director for StratNeuro, KI.
2003–2011: Deputy Chair at the Department of Neuroscience, Karolinska Institutet.
G. Supervision
>10 PhD students
>25 Postdocs
Guest professors:
(Harris-Warrick/Bruce Johnson, Cornell University; Goulding, Salk Institute; Glover, Oslo University; Del Negro William and Mary, US).
H. Membership Editorial Boards and review jobs
Member of editorial boards: Current Opinion of Neurobiology (2008-), Neural Development (2007-2019), Brain Research Bulletin (2000-), Faculty of 1000 (2004-2009). Journal of Neurophysiology (1996-2012).
Reviewing editor: E-Life (2012-2018), EJN (2015-).
Chief Editor: Current Opinion in Neurobiology (2019-)
Associate Editor: Frontiers in Neuroscience (2011-2016).
Section Editor: Current Opinion of Neurobiology, Motor, 2004 and 2015.
I. Selected list of invited talks (last 10 years)
Plenary lectures/Distinguished lectures: Plenary Lecture, Federation of Physiological Society, Bologna (2019); Key note speaker Neuroscience Day University of Miami, US (2021); Opening Lecture, IBRO meeting/Hungarian Society for Neuroscience Budapest (2022); Key Note Lecture, Gordon Conference – Spinal Cord Injury US (2022); Plenary lecture ENCP congress in Vienna (2022); Brain Prize lecture Copenhagen (2022); Plenary Lecture - Regional FENS meeting Portugal (2023); Keynote lecture for the Collaborative Research Center on Motor Control Germany (2023); Opening lecture Neuroscience Day in Aarhus (2023); Plenary lecture Canadian Association of Neuroscience (2023); Plenary lecture International Movement Disorders Conference in Copenhagen (2023). Plenary lecture Nordic Neuroscience meeting (2024).
Recent Meeting organization: In addition to FENS/IBRO/SFN, Kiehn has organized several international meetings on movement: Recent Benzon symposium (Bringing movement circuits together, 2023), Brain Prize/FENS conference on Circuits for Movement 2024.
J. Research grants received during the last 7-8 years
2018-2022: Swedish Medical Research Council: 9MSEK – PI;
2011-2016: ERC – advanced grant (2.5 MEuro) -PI;
2016-2020: ERC – advanced grant (2.5 MEuro) – PI;
2016-2024: Novo Nordisk Laureate grant (40 MDK)- PI;
2018-2022: Lundbeck Collaborative grant – together with Gilberto Fisone KI (10MDK)- PI;
2019-2026: Lundbeck Professorship grant (32MDK) -PI;
Our lab has developed a broad experimental repertoire, that includes:
- Production of transgenic mice lines: Vglut2Cre (Borgius et al. 2010), Vglut2GFP (Calderia et al. 2017), Vglut2ChR2 (Hagglund et al. 2010), Hoxb8Flipo, R26CAGfrt/stop/flxstopGaMP3 tailored for the locomotor experiments.
- Application of genetically driven tracing: trans-synaptic tracing with attenuated rabies/AAV.
- mRNA seq of interneurons in the spinal cord.
- In vitro electrophysiology in isolated spinal cord-brainstem, including calcium- imaging and optogenetics.
- Gait analyses.
- In vivo electrophysiology, optogenetic, chemogenetic, and motor neurobehavioral analyses of acute and chronic perturbations (MotoRater; open field, treadmill etc).
- Clarity-clearing of tissue and imaging.
- In situ hybridization.
These tools provide a solid basis for the sophisticated functional and network studies needed to understand the principal mode of operation of a large-scale mammalian motor circuits.
2023
- Hsu, Li-Ju, Bertho, Maelle Claire Valentine & Kiehn, Ole, 2023. Deconstructing the modular organization and real-time dynamics of mammalian spinal locomotor networks. Nature Communications. 14, 1, 18 p., 873. Contribution to journal › Journal article › Research › peer-review
- Goñi Erro, Haizea, Selvan, Raghav, Caggiano, V., Leiras, Roberto & Kiehn, Ole, 2023. Pedunculopontine Chx10 + neurons control global motor arrest in mice. Nature Neuroscience. 26, p. 1516–1528 43 p. Contribution to journal › Journal article › Research › peer-review
2022
-
Masini D, Kiehn O. Nat Commun (2022) Jan 26. Targeted activation of midbrain neurons restores locomotor function in mouse models of parkinsonism. Nature Communications. 2022 Jan 26;13(1):504. doi: 10.1038/s41467-022-28075-4. PMID:35082287
-
Leiras R, Cregg JM, Kiehn O. Annu Rev Neurosci. 2022 Jan 5. Brainstem Circuits for Locomotion. Annual Review of Neuroscience. 2022 Jan 5. doi: 10.1146/annurev-neuro-082321-025137. Online ahead of print. PMID: 34985919.
2021
-
Zelenin PV, Vemula MG, Lyalka VF, Kiehn O, Talpalar AE, Deliagina TG.J Neurosci (2021) Apr. Differential Contribution of V0 Interneurons to Execution of Rhythmic and Nonrhythmic Motor Behaviors. J NEUROSCI 14;41(15):3432-3445. doi: 10.1523/JNEUROSCI.1979-20.2021. Epub 2021 Feb 26.PMID: 33637562
-
Wu H, Petitpré C, Fontanet P, Sharma A, Bellardita C, Quadros RM, Jannig PR, Wang Y, Heimel JA, Cheung KKY, Wanderoy S, Xuan Y, Meletis K, Ruas J, Gurumurthy CB, Kiehn O, Hadjab S, Lallemend F. Nat Commun. (2021) Feb. Distinct subtypes of proprioceptive dorsal root ganglion neurons regulate adaptive proprioception in mice. Nature Communications. 15;12(1):1026. doi: 10.1038/s41467-021-21173-9. PMID: 33589589 Free PMC article.
- Allodi I, Montañana-Rosell R, Selvan R, Löw P, Kiehn O (2021). Locomotor deficits in a mouse model of ALS are paralleled by loss of V1-interneuron connections onto fast motor neurons. Nature Communications. 12, Article number: 3251 (2021) >NatureComm
2020
- Yuste, R, Hawrylycz, M,...., Kiehn O,..., Lein, E (2020). A community-based transcriptomics classification and nomenclature of neocortical cell types. Nature Neuroscience. Aug 24. 13 s. >NatureNeuro
- Cregg M J, Leiras R, Montalant A, Wanken P, Wickersham R I, Kiehn O (2020). Brainstem neurons that command mammalian locomotor asymmetries. Nature Neuroscience. May 11. DOI: s41593-020-0633-7 >NatureNeuro
- Marcantoni M, Fuchs A, Löw P, Bartsch D, Kiehn O, Bellardita C (2020). Early delivery and prolonged treatment with nimodipine prevents the development of spasticity after spinal cord injury in mice. Science Translational Medicine. Apr 15. DOI: 10.1126/scitranslmed.aay0167 >Scitranslmed
2019
- Allodi I, Nijssen J, Benitez JA, Schweingruber C, Fuchs A, Bonvicini G, Cao M, Kiehn O, Hedlund E (2019). Modeling Motor Neuron Resilience in ALS Using Stem Cells. Stem Cell Reports >ScienceDirect
- Wang Y, Wu H, Zelenin P, Fontanet P, Wanderoy S, Petitpré C, Comai G, Bellardita C, Xue-Franzén Y, Huettl RE, Huber AB, Tajbakhsh S, Kiehn O, Ernfors P, Deliagina TG, Lallemend F, Hadjab S (2019). Muscle-selective RUNX3 dependence of sensorimotor circuit development. Development. 2019 Oct 24;146(20). doi: 10.1242/dev.181750. >pubmed
2018
- Zhang MD, Su J, Adori C, Cinquina V, Malenczyk K, Girach F, Peng C, Ernfors P, Löw P, Borgius L, Kiehn O, Watanabe M, Uhlén M, Mitsios N, Mulder J, Harkany T, Hökfelt T (2018). Ca2+-binding protein NECAB2 facilitates inflammatory pain hypersensitivity. J Clin Invest Jun 12. doi: 10.1172/JCI120913 >pubmed
- Häring M, Zeisel A, Hochgerner H, Rinwa P, Jakobsson J, Lönnerberg P, La Manno G, Sharma N, Borgius L, Kiehn O, Lagerström M, Linnarsson S, Ernfors P (2018). Neuronal atlas of the dorsal horn defines its architecture and links sensory input to transcriptional cell types. Nature Neuroscience Apr 23. >nature
- Bellardita C, Marcantoni M, Löw P, Kiehn O (2018). Sacral Spinal Cord Transection and Isolated Sacral Cord Preparation to Study Chronic Spinal Cord Injury in Adult Mice. Bio Protocol Apr 05. >bio-protocol
- Caggiano V, Leiras R, Goñi-Erro H, Masini D, Bellardita C, Bouvier J, Caldeira V, Fisone G, Kiehn O (2018). Midbrain circuits that set locomotor speed and gait selection. Nature Jan 17. doi: 10.1038/nature25448 http://rdcu.be/EV9n
2017
- Bellardita C, Caggiano V, Leiras R, Caldeira V, Fuchs A, Bouvier J, Löw P, Kiehn O (2017).Spatiotemporal correlation of spinal network dynamics underlying spasms in chronic spinalized mice.Elife. Feb 13;6. pii: e23011>pubmed
- Caldeira V, Dougherty KJ, Borgius L, Kiehn O (2016).Spinal Hb9::Cre-derived excitatory interneurons contribute to rhythm generation in the mouse.Sci Rep. 7: 41369.>pubmed
2016
- Kiehn O (2016).Decoding the organization of spinal circuits that control locomotion.Nat Rev Neurosci,17(4): 224-238.>pubmed
2015
- Bouvier J, Caggiano V, Leiras R, Caldeira V, Bellardita C, Balueva K, Fuchs A, Kiehn O (2015).Descending Command Neurons in the Brainstem that Halt Locomotion.Cell,163(5): 1191-1203.>pubmed
- Kiehn O, Churchland MM (2015).Editorial overview: Motor circuits and action.Curr Opin Neurobiol,33(Aug): v-vi.>pubmed
- Bellardita C, Kiehn O (2015).Phenotypic characterization of speed-associated gait changes in mice reveals modular organization of locomotor networks.Curr Biol,25(11): 1426-1436.>pubmed
- Ruffault PL, D'Autréaux F, Hayes JA, Nomaksteinsky M, Autran S, Fujiyama T, Hoshino M, Hägglund M, Kiehn O, Brunet JF, Fortin G, Goridis C (2015).The retrotrapezoid nucleus neurons expressing Atoh1 and Phox2b are essential for the respiratory response to CO2.Elife,4: 10.7554/eLife.07051.>pubmed
- Shevtsova NA, Talpalar AE, Markin SN, Harris-Warrick RM, Kiehn O, Rybak IA (2015).Organization of left-right coordination of neuronal activity in the mammalian spinal cord: Insights from computational modelling.J Physiol,593(11): 2403-2426.>pubmed
2014
- Zhang MD, Tortoriello G, Hsueh B, Tomer R, Ye L, Mitsios N, Borgius L, Grant G,Kiehn O, Watanabe M, Uhlén M, Mulder J, Deisseroth K, Harkany T, Hökfelt TG (2014).Neuronal calcium-binding proteins 1/2 localize to dorsal root ganglia and excitatory spinal neurons and are regulated by nerve injury.Proc Natl Acad Sci U S A,111(12): E1149-1158.>pubmed
- Borgius L, Nishimaru H, Caldeira V, Kunugise Y, Löw P, Reig R, Itohara S, Iwasato T, Kiehn O (2014).Spinal Glutamatergic Neurons Defined by EphA4 Signaling Are Essential Components of Normal Locomotor Circuits.J Neurosci,34(11): 3841-3853.>pubmed
- Forssberg H, Kiehn O (2014).[2014 Nobel Prize in Physiology or Medicine. The Nobel laureates have explored the internal GPS of the brain].(Article in Swedish).Läkartidningen,111(41): 1766-1767.>pubmed
2013
- Dougherty KJ, Zagoraiou Z, Satoh D, Rozani I, Doobar S, Arber S, Jessell TM, Kiehn O. (2013).Locomotor Rhythm Generation Linked to the Output of Spinal Shox2 Excitatory Interneurons.Neuron,80(4): 920-933.>pubmed
- Rybak IA, Shevtsova NA, Kiehn O. (2013).Modelling genetic reorganizations in the mouse spinal cord affecting left-right coordination during locomotion.J Physiol.591(Pt 22):5491-5508>pubmed
- Talpalar AE, Bouvier J, Borgius L, Fortin G, Pierani A, Kiehn O (2013).Dual-mode operation of neuronal networks involved in left–right alternation.Nature, 500: 85–88.>pubmed
- Hägglund M, Dougherty KJ, Borgius L, Itohara S, Iwasato T, Kiehn O (2013).Optogenetic dissection reveals multiple rhythmogenic modules underlying locomotion.Proc Natl Acad Sci U S A.110(28):11589-94. >pubmed
2011
- Talpalar AE, Endo T, Löw P, Borgius L, Hägglund M, Dougherty KJ, Rygg J, Hnasko TS, Kiehn O (2011).Identification of minimal neuronal networks involved in flexor-extensor alternation in the mammalian spinal cord.Neuron, 71(6):1071-84.>pubmed
- Leão RN, Colom LV, Borgius L, Kiehn O, Fisahn A (2011).Medial septal dysfunction by Aβ-induced KCNQ channel-block in glutamatergic neurons.Neurobiol Aging.2012 Sep;33(9):2046-61.>pubmed
- Restrepo CE, Margaryan G, Borgius L, Lundfald L, Sargsyan D, Kiehn O (2011).Change in the balance of excitatory and inhibitory midline fiber crossing as an explanation for the hopping phenotype in EphA4 knockout mice.European Journal of Neuroscience, 34(7):1102-12.>pubmed
- Kiehn, O (2011).Development and functional organization of spinal locomotor circuits.Current Opinion of Neurobiology, 21(1):100-9>pubmed
2010
- Holz A, Kollmus H, Ryge J, Niederkofler V, Dias J, Ericson J, Stoeckli ET, Kiehn O, Arnold HH. (2010).The transcription factors Nkx2.2 and Nkx2.9 play a novel role in floor plate development and commissural axon guidance.Development, 137(24): 4249-60.>pubmed
- Kiehn O, Dougherty KJ, Hägglund M, Borgius L, Talpalar A, Restrepo CE. (2010)Probing spinal circuits controlling walking in mammals.Biochemical and Biophysical Research Communication, Karolinska Institutet 200 year anniversary, special issue 396(1): 11-18. >pubmed
- Ryge J, Winther O, Wienecke J, Sandelin A, Westerdahl AC, Hultborn H, Kiehn O. (2010).Transcriptional regulation of gene expression clusters in motor neurons following spinal cord injury.BMC Genomics. 11: 365-387.>pubmed
- Borgius L, Restrepo CE, Leao RN, Saleh N, Kiehn O. (2010).A transgenic mouse line for molecular genetic analysis of excitatory glutamatergic neurons.Molecular and Cellular Neuroscience, 45(3):245-257.>pubmed
- Talpalar AE, Kiehn O. (2010)Glutamatergic mechanisms for speed control and networkoperation in the rodent locomotor CPG.Frontiers in Neuroscience: Neural Circuits, (4) pii: 1-14.>pubmed
- Hägglund M, Borgius L, Dougherty KJ, Kiehn O. (2010).Activation of glutamatergic neurons in the mammalian brainstem or spinal cord evoke hind-limp locomotion.Nature Neuroscience, 13(2): 246-52.>pubmed
- Dougherty KJ, Kiehn O. (2010).Functional organization of V2a-related locomotor circuits in the rodent spinal cord.Ann N Y Acad Sci, 1198:85-93.>pubmed
- Dougherty KJ, Kiehn O. (2010).Firing and cellular properties of V2a interneurons in the rodent spinal cord.Journal of Neuroscience, 30(1): 24-37.>pubmed
- Wienecke J, Westerdahl A-C, Hultborn H, Kiehn O. Ryge J, (2010).Global gene expression analysis of rodent motor neurons following spinal cord transaction associate molecular mechanisms to the development of post-injury spasticity.Journal of Neurophysiology, 103(2): 761-78.>pubmed
2009
- Restrepo, E, Lundfald, Szabó, LG F. Erdélyi, F, H.U. Zeilhofer, HU, Glover JC, Kiehn O (2009).Transmitter phenotype of commissural interneurons in the rodent spinal cord.Journal of Comparative Neurology, 517 (2): 177-193.>pubmed
2008
- Endo T, and Kiehn O. (2008).Asymmetric operation of the locomotor central pattern generator in the neonatal mouse spinal cord.Journal of Neurophysiology,100(6): 3043-3054.>pubmed
- Ryge J, Westerdahl AC, Alstrøm P, and Kiehn O. (2008).Gene expression profiling of two distinct neuronal populations in the rodent spinal cord.PLoS ONE, 3(10): e3415-25.>pubmed
- Crone, S, Quinlan, KA, Zagoraiou, L, Droho, S, Restrepo, CE, Lundfald, L Setlak J, Jessell TM, Kiehn# O and Sharma, K# (2008).Selective ablation of ipsilateral excitatory interneurons decouples left-right locomotor coordination.Neuron, 60(1): 70-83. # shared last author.>pubmed
- Kiehn O, Quinlan KA, Restrepo CE, Lundfald L, Borgius L, Talpalar AE, Endo T. (2008).Excitatory components of the mammalian locomotor CPG.Brain Research Review, 57(1): 56-63.>pubmed
2007
- Lundfald L, Restrepo CE, Butt SJ, Peng CY, Droho S, Endo T, Zeilhofer HU, Sharma K, Kiehn O (2007).Phenotype of V2-derived interneurons and their relationship to the axon guidance molecule EphA4 in the developing mouse spinal cord. European Journal of Neuroscience. 26(11): 2989-3002.>pubmed
- Quinlan KA, Kiehn O. (2007).Segmental, synaptic actions of commissural interneurons in the mouse spinal cord.Journal of Neuroscience, 27(24): 6521-30.>pubmed
- Wallen, P., Grillner S., and Kiehn, O. (2007). Nätverk styr våra rörelser.Pp 75-90. Hjärnan (ed. Olson, L). Karolinska Institutet, University Press.
2006
- Kiehn O (2006).Locomotor circuits in the mammalian spinal cord.Annual Review in Neuroscience, 29: 279-306.>pubmed
- Nishimaru, H, Restrepo CE, Kiehn, O. (2006).Activity of Renshaw cells during locomotor-like rhythmic activity in the isolated spinal cord of neonatal mice.Journal of Neuroscience, 26(20): 5320-8.>pubmed
- Gosgnach S, Lanuza GM, Butt SJB, Saueressig H, Ying Zhang Y, Velasquez T, Callaway E, Kiehn O, Goulding M (2006).V1 spinal neurons regulate the speed of vertebrate locomotor outputs.Nature, 440(7081): 215-9.>pubmed
2005
- Butt SJB, Lundfald L, Kiehn O (2005)EphA4 defines a class of excitatory locomotor-related interneurons.PNAS,102(39): 14098-103. >pubmed
- Egea J, Nissen UV, Dufour A, Sahin M, Greer P, Kullander K, Mrsic-Flogel TD, Greenberg ME, Kiehn O, Vanderhaeghen P, Klein R. (2005).Regulation of EphA4 Kinase activity is required for a subset of axon guidance decisions suggesting a key role for receptor clustering in Eph function.Neuron, 47(4): 515-528.>pubmed
- Lee IH, Lindqvist E, Kiehn O, Widenfalk J, Olson L. (2005)Glial and neuronal connexin expression patterns in the rat spinal cord during development and following injury.Journal of Comparative Neurology, 489(1): 1-10.>pubmed
- Nishimaru H, Restrepo CE, Ryge J, Yanagawa Y, Kiehn O (2005)Mammalian motor neurons corelease glutamate and acetylcholine at central synapses.PNAS, 102(14): 5245-5249.>pubmed
2004
- Kiehn O, Kullander K. (2004).Central pattern generators deciphered by molecular genetics.Neuron,41(3): 317-21.>pubmed
2003
- Butt S.J.B., Kiehn O. (2003).Functional identification of interneurons responsible for left-right coordination of hindlimbs in mammals.Neuron,38(6): 953-63.>pubmed
- Birinyi A., Viszokay K., Weber I, Kiehn O., Antal, M (2003).Synaptic targets of commissural interneurons in the lumbar spinal cord of neonatal rats.Journal of Comparative Neurology, 467: 429-40.>pubmed
- Kiehn O, Butt SJ. (2003).Physiological, anatomical and genetic identification of CPG neurons in the developing mammalian spinal cord.Progress in Neurobiology,70(4): 347-61.>pubmed
- Kullander K. , Butt S.J.B., Lebret J., Lundfald L., Restrepo E., Klein R., Kiehn, O. (2003).Role of EphA4 and EphrinB3 in local circuits that control walking.Science, 299: 1889-1892.>pubmed
- Merrywest, S., McDearmid J., Kjaerulff O., Kiehn O., Sillar, KT. (2003).Mechanisms underlying the noradrenergic modulation of longitudinal co-ordination during swimming in Xenopus lavevis tadpoles.European Journal of Neuroscience, 17:1013-1022.>pubmed
2002
- Butt SJB, Lebret J, Kiehn O. (2002).Organization of left right alternation in the mammalian locomotor network.Brain Research Review, 40 (1-3): 107-117.>pubmed
- Kiehn, O. and Tresch, MC (2002).Gap junctions and motor behavior. Trends in Neuroscience,25(2): 108-115.>pubmed
- Butt SJ, Harris-Warrick RM, Kiehn O. (2002).Firing properties of identified interneuron populations in the mammalian hindlimb central pattern generator.Journal of Neuroscience, 22(22): 9961-9971.>pubmed
- Tresch MC, Kiehn O. (2002).Synchronization of motor neurons during locomotion in the neonatal rat: predictors and mechanisms.Journal of Neuroscience, 22(22): 9997-10008.>pubmed
- Stokke MF, Nissen UV, Glover JC., Kiehn O. (2002).Projection patterns of commissural interneurons in the lumbar spinal cord of the neonatal rat.Journal Comparative Neurology, 446(4): 349-359.>pubmed
2001
- Beierholm U, Nielsen CD, Ryge J, Alstrom P, Kiehn O. (2001).Characterization of reliability of spike timing in spinal interneurons during oscillating inputs.Journal of Neurophysiology, 86(4): 1858-1868.>pubmed
- Kjaerulff O, Kiehn O. (2001).5-HT modulation of multiple inward rectifiers in motoneurons in intact preparations of the neonatal rat spinal cord.Journal of Neurophysiology, 85(2): 580-593.>pubmed
2000
- Tresch MC, and Kiehn O. (2000). Population reconstruction of the locomotor cycle from interneuron activity in the mammalian spinal cord.Journal Neurophysiology, 83(4): 1972-1978.>pubmed
- Gorassini M, Eken T, Bennett DJ, Kiehn O, and Hultborn H. (2000).Activity of hindlimb motor units during locomotion in the conscious rat.Journal of Neurophysiology, 83(4): 2002-2011.>pubmed
- Raastad M, and Kiehn O. (2000).Spike coding during locomotor network activity in ventrally located neurons in the isolated spinal cord from neonatal rat.Journal of Neurophysiology, 83(5): 2825-2834.>pubmed
- Tresch MC, and Kiehn O. (2000).Motor coordination without action potentials in the mammalian spinal cord.Nature Neuroscience, 3(6): 593-599.>pubmed
- Kiehn O, Kjaerulff O, Tresch MC, and Harris-Warrick RM. (2000).Contributions of intrinsic motor neuron properties to the production of rhythmic motor output in the mammalian spinal cord.Brain Research Bulletin, 53(5): 649-659.>pubmed
1999
- Gorassini M, Bennett DJ, Kiehn O, Eken T, and Hultborn H. (1999)Activation patterns of hindlimb motor units in the awake rat and their relation to motoneuron intrinsic properties.Journal of Neurophysiology, 82(2): 709-717.>pubmed
- Kiehn O, Sillar KT, Kjaerulff O, and McDearmid JR. (1999).Effects of noradrenaline on locomotor rhythm-generating networks in the isolated neonatal rat spinal cord.Journal of Neurophysiology, 82(2): 741-746.>pubmed
- Tresch MC, and Kiehn O. (1999).Coding of locomotor phase in populations of neurons in rostral and caudal segments of the neonatal rat lumbar spinal cord.Journal of Neurophysiology, 82(6): 3563-3574.>pubmed
- Eide AL, Glover J, Kjaerulff O, and Kiehn O. (1999).Characterization of commissural interneurons in the lumbar region of the neonatal rat spinal cord.Journal of Comparative Neurology,403(3): 332-345.>pubmed
1998
- Kiehn O, and Eken T. (1998).Functional role of plateau potentials in vertebrate motor neurons.Current Opinion Neurobiology, (6): 746-52.>pubmed
- Kiehn O, and Kjaerulff O. (1998)Distribution of central pattern generators for rhythmic motor outputs in the spinal cord of limbed vertebrates.Ann N Y Acad Sci. 860:110-129.>pubmed
- Raastad M, Enríquez-Denton M, and Kiehn O. (1998).Synaptic signaling in an active central network only moderately changes passive membrane properties.PNAS, 95(17): 10251-6.>pubmed
1997
- Kjaerulff, O. and Kiehn, O. (1997).Crossed synaptic inpus to motoneurons during selective activation of the contralateral locomotor network.Journal of Neuroscience, 17(24): 9433-9447.>pubmed
- Kiehn, O. and Eken, T. (1997).Prolonged firing in motor units - evidence of plateau potentials in human motoneurons?Journal of Neurophysiology, 78(6): 3061-3068.>pubmed
- Booth, V. Rinzel, J. and Kiehn, O. (1997).A compartmental model of vertebrate motoneurons for Ca2+-dependent spiking and plateau potentials under pharmacological treatment.Journal of Neurophysiology, 78(6): 3371-3385.>pubmed
- Raastad, M., Johnson, B.R. and Kiehn O. (1997).An analysis of excitatory and inhibitory postsynaptic currents carrying information about rhythmic activity in the isolated spinal cord from neonatal rats.Journal of Neurophysiology, 78(4): 1851-1859.>pubmed
- Iizuka, M., Kiehn, O. and Kudo, N. (1997).Development of sensory resetting of 5-HT and NMDA-induced locomotion in neonatal rats.Experimental Brain Research,114. 193-204.>pubmed
1996
- Raastad, M. Johnson, B.R. and Kiehn O. (1996).The number of single postsynaptic currents necessary to produce locomotor-related cyclic information in neurons in the neonatal rat spinal cord.Neuron, 729-738.>pubmed
- Kiehn, O., Johnson, B.R. and Raastad, M. (1996).Plateau properties in mammalian spinal interneurons during transmitter-induced locomotor activity.Neuroscience, 75 (1): 263-273.>pubmed
- Kjærulff, O. and Kiehn, O. (1996).Distribution of Networks generating and coordinating locomotor activity in the neonatal rat spinal cord in vitro. A lesion study.Journal of Neuroscience,16(8): 5777-5794.>pubmed
- Kiehn, O. and Kjærulff, O. (1996).Spatiotemporal characteristics of 5-HT and dopamine-induced hindlimb locomotor activity in the in vitro neonatal rat.Journal of Neurophysiology, 75(4): 1472-1482.>pubmed
- Kiehn, O., Erdal, J. Eken, T. and Bruhn, T. (1996).Selective depletion of spinal monoamines changes the rat soleus EMG from a tonic to a more phasic pattern.Journal of Physiology, 492 (1): 173-184.>pubmed
1994
- Kjærulff, O., Barajon, I. and Kiehn, O. (1994).Distribution of sulforhodamine labelled cells in the neonatal rat spinal cord following chemically induced locomotor activity in vitro.Journal of Physiology, 478 (2): 265-273.>pubmed
1993
- Nielsen, J., Petersen, N., Ballegaard, M., Biering-Sørensen, F. and Kiehn, O. (1993).H-reflexes are less depressed following muscle stretch in spastic spinal cord injured patients than in healthy subjects.Experimental Brain Research,97: 173-176.>pubmed
- Hounsgaard, J. and Kiehn, O. (1993).Calcium spikes and calcium plateaux evoked by differential polarization in dendrites of turtle motoneurones in vitro.Journal of Physiology, 468: 245-259.>pubmed
1992
- Hultborn, H. and Kiehn, O. (1992).Neuromodulation of vertebrate motoneurone membrane properties. Current Opinion in Neurobiology, 2: 770-775.>pubmed
- Kiehn, O., Iizuka, M. and Kudo, N. (1992).Resetting from low threshold afferents of N-methyl-D-aspartate-induced locomotor rhythm in the isolated spinal cord-hindlimb preparation newborn rats.Neuroscience Letters, 148: 43-46.>pubmed
- Kiehn, O. and Hultborn, H. and Conway, B. (1992).Rhythmic spinal locomotor activity in acutely spinalized cats induced by intrathecal application of noradrenaline.Neuroscience Letters, 143: 243-246.>pubmed
- Kiehn, O., Rostrup, E and Møller, M. (1992).Monoaminergic systems in the brainstem and spinal cord of the turtle Pseudemys scripta elegans as revealed by antibodies against serotonin and tyroxin hydroxylase.Journal of Comparative Neurology, 325(4): 527-547.>pubmed
- Kiehn, O. and Harris-Warrick, R. (1992).5-HT modulation of hyperpolarization-activated current and calcium-dependent outward current in a Crustacean motoneurone.Journal of Neurophysiology, 68 (2): 496-508.>pubmed
- Kiehn, O. and Harris-Warrick, R. (1992).Serotonergic stretch receptors induce plateau properties in a Crustacean motoneurone by a dual conductance mechanism. Journal of Neurophysiology, 68 (2): 485- 495.>pubmed
1991
- Kiehn, O. (1991).Plateau potentials and active integration in the 'final common pathway' for motor behaviour.Trends in Neurosciences, 14 (2): 68-73.>pubmed
1989
- Hounsgaard, J. and Kiehn, O. (1989).Serotonin induced bistability of turtle motoneurones caused by a nifedipine sensitive plateau potential.Journal of Physiology 414: 265-282.>pubmed
- Eken, T., Hultborn, H. and Kiehn, O. (1989). Possible function of transmitter-controlled plateau potentials in alfa-motoneurones.Progress in Brain Research, 257-267.>pubmed
- Eken, T. and Kiehn, O. (1989).Bistable firing properties of soleus motor units in freely moving rats.Acta Physiologica Scandinavica, 136 (3): 383-394.>pubmed
1988
- Conway, B.A., Hultborn, H., Kiehn, O. and Mintz, I. (1988).Plateau potentials in alpfa-motoneurones induced by intravenous injection of DOPA and clonidine in the spinal cat.Journal of Physiology, 405: 369-384.>pubmed
- Hounsgaard, J., Hultborn, H., Jespersen, B. and Kiehn, O. (1988).Bistability of alpfa-motoneurones in the decerebrate cat and in the acute spinal cat after intravenous 5-hydroxytryptophan.Journal of Physiology, 405: 345-367.>pubmed
- Crone, C., Hultborn, H., Kiehn, O., Mazieres, L. and Wigström, H. (1988).Maintained changes in motoneuronal excitability by short-lasting synaptic inputs in the decerebrate cat.Journal of Physiology 405: 321-343.>pubmed
- Hounsgaard, J., Kiehn, O. and Mintz, I. (1988).Response properties of motoneurones in a slice preparation of the turtle spinal cord.Journal of Physiology, 398: 575-589.>pubmed
1987
- Conway, B.A., Hultborn, H. and Kiehn, O. (1987).Proprioceptive input resets central locomotor rhythm in the spinal cat.Experimental Brain Research, 68: 643-656.>pubmed
1986
- Hounsgaard, J., Hultborn, H. and Kiehn, O. (1986).Transmitter-controlled properties of alfa-motoneurones causing a long-lasting motor discharge to brief excitatory inputs.Progress in Brain Research, 64: 39-49.>pubmed
1985
- Hounsgaard, J. and Kiehn, O. (1985).Ca++ dependent bistability by serotonin in spinal motoneurones.Experimental Brain Research, 57: 422-425.>pubmed
1984
- Hounsgaard, J., Hultborn, H., Jespersen, B. and Kiehn, O. (1984).Intrinsic membrane properties causing a bistable behaviour of alpfa-motoneurones.Experimental Brain Research, 55: 391-394.>pubmed
1983
- Hansen T, Kiehn O, Kristensen J, Larsen JE, Lorenzen T, Pociot F, Rathcke M, Norn M. (1983).Schirmer’s tåretest.Ugeskr Laeger. 145(34): 2573-5.>pubmed
Guest Editor
- Brains Research Reviews (2008) – Special issue Vol 57(1):Networks in Motion.Editors Grillner S, El Manira A, Kiehn O, Rossignol S, Stein P.
- Current Opinions of Neurobiology (2002),Motor Section. Editors: Anderson and Kiehn.
- Brains Research Reviews (2002) – Special issue Vol. 40 (1-3). Editors: Schouenborg J., Kiehn O., Garwic M., Grillner S., Hultborn H.
- Kiehn, O. and Midtgaard, J. (1996).Transmission and processing of synaptic information in single cells.Acta Physiologica Scandinavica 157(3), 367-368.
- Annals of the New York Academy of Sciences (1998).Neuronal Mechanisms for Generating Locomotor Activity. Editors Kiehn O, Harris-Warrick RM, Jordan LM, Hultborn H, Kudo N.>link
Book chapters
- Ole Kiehn O, Dougherty K (2013).Locomotion: Circuits and Physiology.In: Neuroscience in the 21st Century (D.W. Pfaff ed.), chapter 38. Published by Springer Science+Business Media.
- Kiehn, O (2010).Locomotor Circuits in the Developing Rodent Spinal Cord.In: Brain Microcircuits (Shepherd, G.M. and Grillner, S., eds.), chapter 37. Published by Oxford University Press.
- Kiehn, O (2008).Rhythmic Movements.Field review. "Encyclopedia of Neuroscience", Springer, Germany.>link
- Kiehn O. et al (2006).Microcircuits in motor systems.77-105- In "Microcircuits: The interface between neurons and global brain function" (eds. Grillner et al.). Status report from Dahlem Conference 2005 – Kiehn Rapporteur. MIT press.
- Kiehn, O. and Katz, P.S (1998).Making Circuits Dance: Modulation of Motor Pattern Generation.In "Neuromodulation" (ed. Katz, P.S), chapter 7. Published by Oxford University Press.
- Kiehn, O., Hounsgaard, J. and Sillar, K.T. (1997).Basic buildings blocks of vertebrate central pattern generators.In: Neurons, Networks and Motor Behavior (Stein, P.S.G., Grillner, S., Selverston, A. and Stuart, D.G., eds.), chapter 9, pp. 47-59 MIT Press, Cambrige, USA.
- Sillar, K.T., Kiehn, O. and Kudo, N. (1997).Chemical modulation of vertebrate motor circuits.In: Neurons, Networks and Motor Behavior (Stein, P.S.G., Grillner, S., Selverston, A. and Stuart, D.G., eds.), chapter 19, pp. 183-193. MIT Press, Cambrige, USA.
- Kiehn, O. and Kjærulff, O. (1995).Organization of spinal locomotor networks and their afferent control in the neonatal rat.In Neural Control of Movements, (Ferrel W.B and Proske, U. eds.), Plenum Publishing, pp. 179-187.
- Kiehn, O. (1993).Transmitter-modulation and possible function of plateau properties in mammalian motoneurones.Physiological Society Magazine (London) 8, 35-38.
- Harris-Warrick, R.M., Flamm, R.E., Johnson, B.R., Katz, P.S., Kiehn, O. and Zhang, B. (1992).Neuromodulation of the small networks in Crustacean.In Proceedings of Neurotox '91, (Duce, I.R. ed), pp. 305-321. Elsevier Science Publishers, Amsterdam.
- Kiehn, O. (1991).Electrophysiology of 5-HT on vertebrate motoneurones.In: Aspects of Synaptic Transmission vol 1: LTP, Galanin, Opiods, Autonomic and 5-HT ed. T. Stone, chapter 18, pp. 527-555. Taylor, London.
- >link
Doctoral Thesis
- Kiehn, O. (1990).Monoaminergic regulation of plateau potentials in vertebrate spinal motoneurones - basic mechanisms and possible functional role.Eget forlag, 1-40.
General papers about Neurobiology
- Wallen, P., Grillner S., andKiehn, O.(2007).Nätverk styr våra rörelser.Pp 75-90. Hjärnan (ed. Olson, L). Karolinska Institutet, University Press.
- Kiehn, O.(1996).Funktionelle studier af rygmarvens gangnetværk under udvikling.Novos årsskrift 1995, 8-11.
- Kiehn, O. (1994).GÅ IND I HJERNEN.WeekendAvisens forskningsside 25-29 marts, 20.
- Jahnsen, H. andKiehn, O. (1989).Nysyn på hjernen.Naturkampen 51, 9-13.
Lab members
| Name | Title | Job responsibilities | |
|---|---|---|---|
| Search in Name | Search in Title | Search in Job responsibilities | |
| Anna-Sofie Hansen | Laboratory Assistant | Kiehn Lab |
|
| Beck Strohmer | Postdoc | Allodi Lab |
|
| Daniel Alejandro Jercog | Assistant Professor | Kiehn Lab |
|
| Divya Rao | Postdoc | Kiehn Lab |
|
| Erik Justin Courcelles | PhD Fellow | NAD |
|
| Giacomo Sitzia | Postdoc | Kiehn Lab |
|
| Ilary Allodi | Guest Researcher | Allodi Lab |
|
| Ima Mustafic | Special Consultant | Kiehn lab |
|
| Iryna Vesth-Hansen | Laboratory Technician | Bellardita Lab, Kiehn lab |
|
| Li-Ju Hsu | Guest Researcher | Kiehn Lab |
|
| Maelle Claire Valentine Bertho | Guest Researcher | Kiehn Lab |
|
| Nathalie Krauth | Assistant Professor | Kiehn Lab |
|
| Ole Kiehn | Professor | Kiehn lab |
|
| Roberto Leiras Gonzalez | Assistant Professor | Kiehn lab |
|
| Simona Agata Maria Bucolo | Academic Research Staff | Kiehn Lab |
|
| Simrandeep Kaur Sidhu | PhD Fellow | Kiehn Lab |
|
| Stephan Dietrich | Postdoc | Kiehn Lab |
|

