Allodi Lab
The Allodi group studies the pathophysiology of Amyotrophic Lateral Sclerosis and Frontotemporal Dementia by investigating inhibitory/excitatory changes in the affected neuronal circuits with the aim to find potential treatments and early indicators for pre-diagnosis.

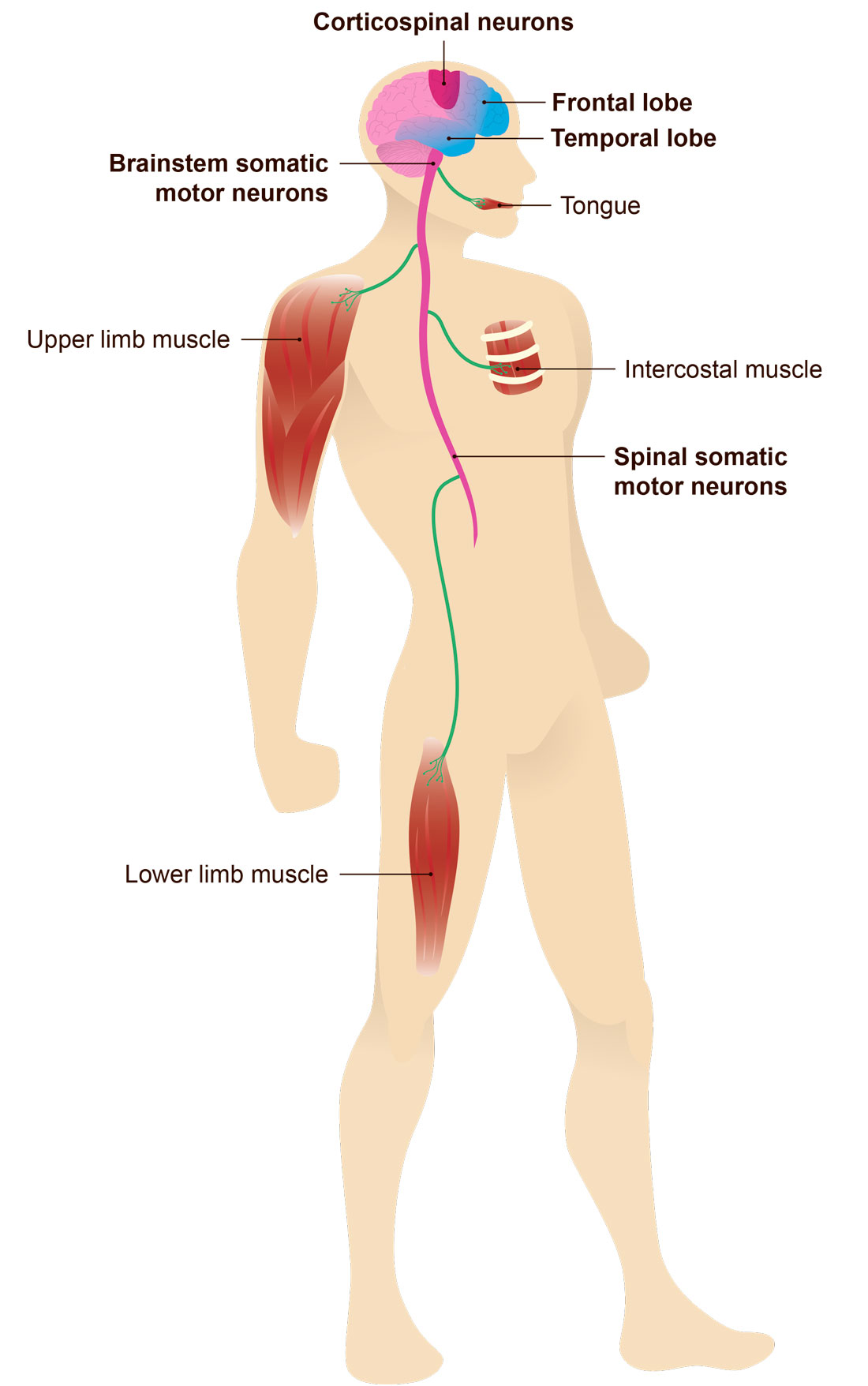
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disorder affecting motor neurons found in spinal cord and brainstem, as well as cortico-spinal neurons. ALS is characterized by muscle wasting and progressive paralysis. Frontotemporal dementia (FTD) is a progressive neurodegenerative condition characterized by the degeneration of neurons in the frontal and temporal lobes, and is characterized by changes in behavior and personality, frontal executive deficits and language dysfunctions. ALS and FTD have historically been considered unrelated neurodegenerative diseases, however it is now clear that genetic mutations in transactive response DNA-binding protein 43 (TARDBP), Fused-in-Sarcoma (FUS) and C9orf72 locus, are associated to both disorders and up to 50% of ALS cases develops FTD. These recent discoveries have transformed the approach to the investigations on disease mechanisms, however the origin and progression of ALS and FTD remain largely unknown and curative therapies do not exist.
Dysfunctional cortical inhibition, leading to excitotoxicity, has been previously reported in both ALS and FTD. Our recent findings (Allodi et al 2021, Nature Communications) obtained in a mouse model of ALS also showed loss of inhibitory inputs in the spinal cord already during asymptomatic stages. Loss of inhibitory inputs can lead to aberrant neuron excitability, intracellular ion dysregulation and cell death, extensively reported in ALS-FTD. However, the exact role of dysfunctional inhibition in the onset and progression of the disease still remains unclear.
We investigate ALS-FTD pathophysiology with a system neuroscience approach, to unravel the neural circuits affected in disease, and to find early indicators of disease for pre-diagnosis. Spatial transcriptomics, multiplexing techniques, gene therapy and machine learning-based behavioral analysis are applied to understand the roles of interneurons in disease and to find new targets for treatment.
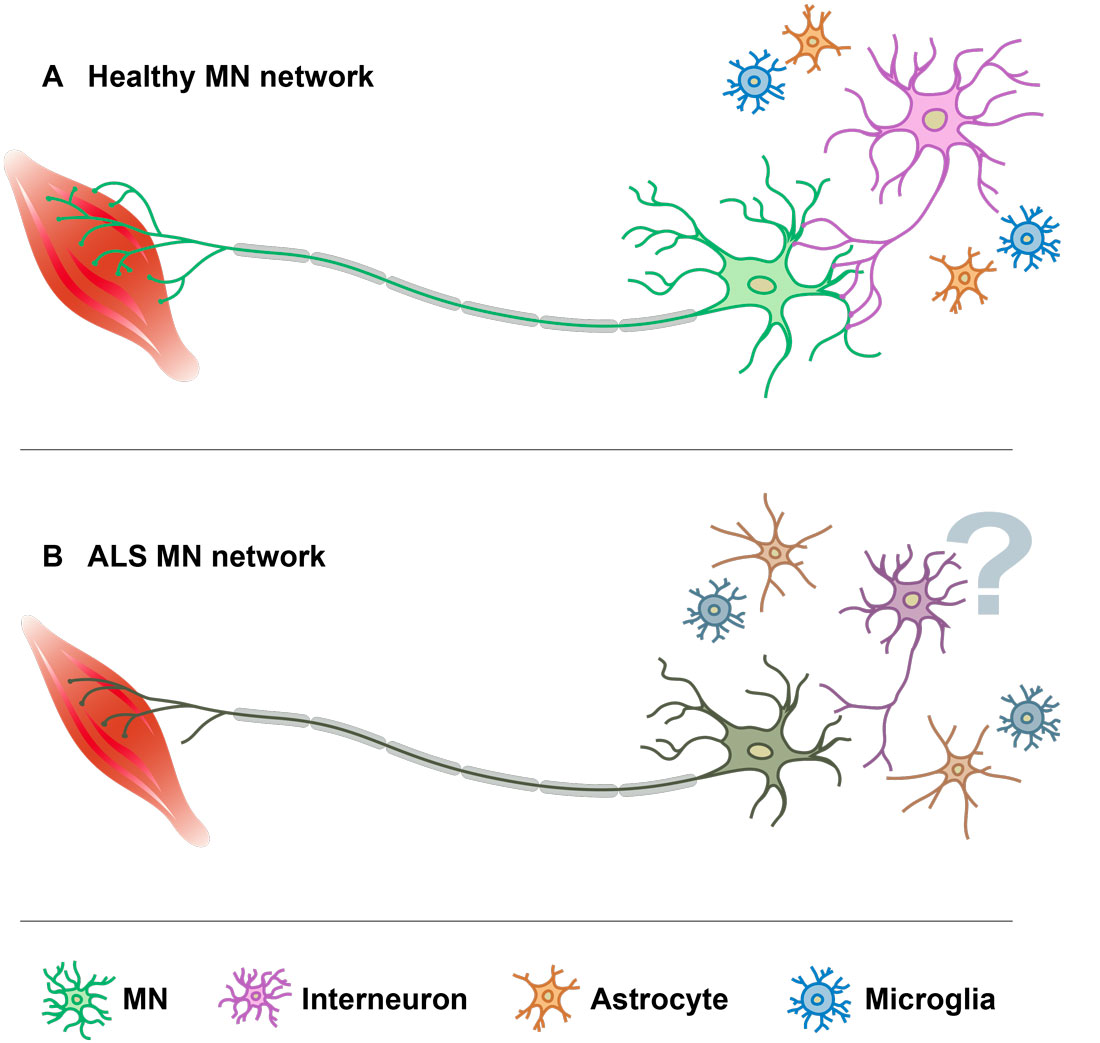
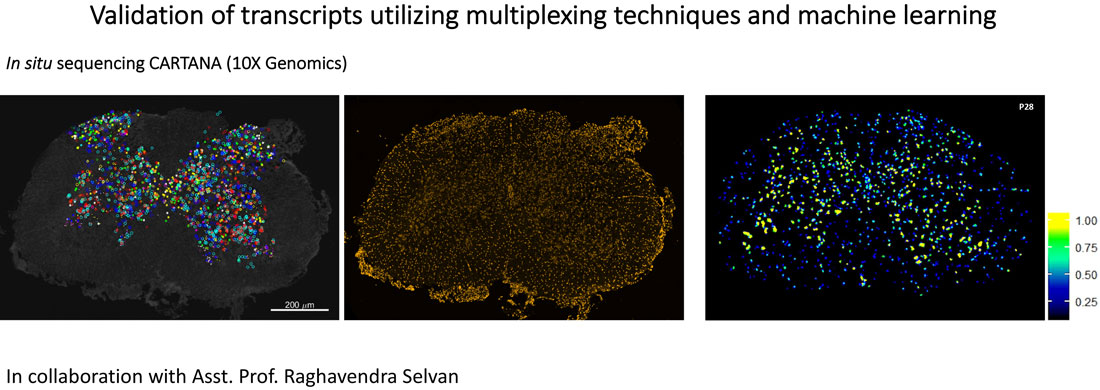
Mora S & Allodi I (2023). Neural circuit and synaptic dysfunctions in ALS-FTD pathology. Frontiers in Neural Circuits (in press).
Mora S, von Huth Friis R, Stuckert A, Noes-Holt G, Motañana-Rosell R, Toft Sørensen A, Selvan R, Allodi I (2023). Stabilization of V1 interneuron-motor neuron connectivity ameliorates motor phenotype in a mouse model of ALS. Pre-print at doi: https://doi.org/10.1101/2022.12.15.520568
Allodi I, Montañana-Rosell R, Selvan R, Löw P, Kiehn O. Loss of V1 interneuron synaptic inputs onto fast fatigable motor neurons leads to gait impairment in a SOD1G93A mouse model. Nature Communications. 12(1),1-18
Allodi I, Nijssen J, Aguila Benitez J, Schweingruber C, Fuchs A, Bonvicini G, Cao M, Kiehn O, Hedlund E. (2019) Modeling motor neuron resilience in ALS using stem cells. Stem Cell Reports 12(6) 1329-41.
Allodi I, Comley LH, Nichterwitz S, Nizzardo M, Simone C, Corti S, Hedlund E. (2016) Differential neuronal vulnerability identifies IGF-2 as a protective factor in ALS and SMA. Scientific Reports 16;6:25960.
Treating ALS disease by rescuing interneuron-motor neuron synaptic connectivity
In ALS, spinal inhibitory interneurons lose their connections to motor neurons during early pre-symptomatic stages before motor neuron death. The loss of these synaptic inputs appears to be restricted to vulnerable motor neurons and triggers pathological events such as, increased intracellular Ca2+ levels, ER stress and cell death. This project aims to rescue the synaptic connections between interneurons and motor neurons in an ALS mouse model by gene therapy, to understand if this alone could reverse the symptoms. This project is conducted in collaboration with Prof. Joost Verhaagen’s laboratory at the Netherlands Institute for Neuroscience and funded by Lundbeckfonden (IA).
Investigations of early dysfunctions in spinal circuits and their contribution to ALS
The loss of synaptic inputs detected in pre-symptomatic ALS mice might be due to alterations occurring either in the inhibitory interneurons or in the motor neurons. To understand the temporal dynamics of this event, single cell transcriptomics combined with electrophysiological analysis will be conducted on spinal inhibitory interneurons. This project is conducted in collaboration with Prof. Marco Beato’s laboratory at UCL (UK) and Prof. Ole Kiehn at University of Copenhagen, and is funded by the Lundbeckfonden (IA), Louis-Hansen Fonden (IA), the Wellcome Trust (MB) and the Novo Nordisk Fonden (OK).
Facing Dementia: Investigating early onset in a mouse model of Frontotemporal Dementia via Facial Expression Analysis
Artificial intelligence (AI)-based methods are applied to identify emotions in a mouse model of FTD in response to positive or adverse stimuli delivered by a virtual reality (VR) interface. Correlations between early FTD symptoms and alterations in the activity of neural circuits found in the frontal and prefrontal lobes of the brain are investigated to find new targets for treatment. This project is conducted in collaboration with Assistant Prof. Raghavendra Selvan and Prof. Erik Dam at the Machine Learning Section, Department of Computer Science (University of Copenhagen).
PROFESSIONAL APPOINTMENTS
2023 Lecturer in Systems Neuroscience, University of St Andrews, UK
2021 Group leader - Assistant Professor, University of Copenhagen, Denmark
2018 Postdoc, University of Copenhagen, Denmark - Kiehn laboratory
2012 Postdoc, Karolinska Institutet, Sweden - Hedlund laboratory
2008 PhD student, Universitat Autonoma de Barcelona, Spain - Navarro laboratory
EDUCATION
2012 PhD degree in Neuroscience, Marie Curie Initial Training Network, UAB (Spain). Obtained: summa cum laude and European mention.
2008 Master’s degree in Psychobiology of motivational and affective processes, Faculty of Psychology, University of Turin (Italy). Obtained: summa cum laude.
2006 Bachelor’s degree in Neuropsychology, Faculty of Psychology, University of Turin (Italy). Obtained first class.
SCIENTIFIC AWARDS and HONORS
2022-2026 Selected scientist for the Lundbeck Foundation Investigator Network (Denmark)
2018 Lundbeck postdoctoral fellowship
2017 Selected scientist for the School of Health Innovation and Entrepreneurship (Karolinska Institutet, University of Oslo and NTNU)
2015 Svenska Sällskapet för Medicinsk Forskning postdoctoral fellowship
2013 Honor Award to the best PhD thesis of the Institute of Neuroscience, UAB, Spain
2008 Marie Curie Initial Training Network PhD fellowship
2008 Gold medal, best thesis of the Faculty of Psychology, University of Turin, Italy
- Mouse models of disease
- Multiplexing techniques: In situ sequencing, RNAscope (v2, HiplexUP)
- Transcriptomics: Nanostring GeoMX spatial profiling and Smart-seq2
- Bioinformatics and computational image analysis
- Behavioral assessment
- Gene therapy
- Viral tracing
- CLARITY techniques and imaging
- In vitro disease modelling (stem cell-derived organoids)
- Calcium imaging
2023
Mora S & Allodi I (2023). Neural circuit and synaptic dysfunctions in ALS-FTD pathology. Frontiers in Neural Circuits (in press).
2022
Mora S, von Huth Friis R, Stuckert A, Noes-Holt G, Motañana-Rosell R, Toft Sørensen A, Selvan R, Allodi I (2023). Stabilization of V1 interneuron-motor neuron connectivity ameliorates motor phenotype in a mouse model of ALS. Pre-print at doi: https://doi.org/10.1101/2022.12.15.520568
2021
Jensen DB, Kadlecova M, Allodi I, Meehan CF. (2021) Response to Letter to Editor on the article Jensen DB, Kadlecova M, Allodi I, Meehan CF (2020). Journal of Physiology https://doi.org/10.1113/JP281539
*Allodi I, Montañana-Rosell R, Selvan R, Löw P, Kiehn O. Loss of V1 interneuron synaptic inputs onto fast fatigable motor neurons leads to gait impairment in a SOD1G93A mouse model. Nature Communications. 12(1),1-18 *First and co-corresponding author.
Kaminski JM, Allodi I, Montañana-Rosell R, Selvan R, Kiehn O. (2021) Deep ensemble model for segmenting microscopy images in the presence of limited labeled data. Peer-reviewed conference proceeding. Link at: https://openreview.net/forum?id=PLSdnHPx-W6
Bolivar S, Allodi I, Herrando M, Udina E. (2021) Evaluation of positive or adverse effects on motor and sensory neurite outgrowth in spinal cord organotypic slices and DRG explants culture. Book chapter, Experimental Neurotoxicology Methods. Vol. 172, ISBN 978-1-0716-1637-6.
2020
Jensen DB, Kadlecova M, Allodi I, Meehan CF. (2020) Spinal motoneurones are intrinsically more responsive in the adult G93A SOD1 mouse model of Amyotrophic Lateral Sclerosis. Journal of Physiology https://doi.org/10.1113/JP280097
Nichterwitz S, Nijssen J, Storvall H,Comley LH, Allodi I, van der Lee M, Schweingruber C, Deng Q, Sandberg R, Hedlund E. (2020) LCM-seq reveals unique transcriptional adaptation mehanisms of resistant neurons in spinal muscular atrophy. Genome Research 30(8) 1083-96.
Nizzardo M, Taiana M, Rizzo F, Benitez JC, Nijssen J, Allodi I, Melzi V, Bresolin N, Comi GP, Hedlund E, Corti S. (2020) Synaptotagmin 13 is neuroprotective across motor neuron diseases. Acta Neuropathologica 1-17.
2019
Allodi I, Nijssen J, Aguila Benitez J, Schweingruber C, Fuchs A, Bonvicini G, Cao M, Kiehn O, Hedlund E. (2019) Modeling motor neuron resilience in ALS using stem cells. Stem Cell Reports 12(6) 1329-41.
Cheng AJ, Allodi I, Chaillou T, Schlittler M, Ivarsson N, Lanner JT, Thams S, Hedlund E, Andersson DC. (2019) Intact single muscle fibres from SOD1G93A ALS mice display preserved specific force, fatigue resistance, and training-like adaptations. Journal of Physiology 597(12) 3133-46.
2018
Mills R, Taylor-Weiner H, Correia JC, Agudelo LZ, Allodi I, Kolonelou C, Martinez-Redondo V, Nichterwitz S, Comley LH, Lundin V, Hedlund E, Ruas JL, Teixeira AI. (2018) Neurturin is a PGC-1α1-controlled myokine that promotes motor neuron recruitment and neuromuscular junction formation. Mol Metabol 7:12-22.
2016
Allodi I, Comley LH, Nichterwitz S, Nizzardo M, Simone C, Corti S, Hedlund E. (2016) Differential neuronal vulnerability identifies IGF-2 as a protective factor in ALS and SMA. Scientific Reports 16;6:25960.
Torres-Espín A, Allodi I, Santos D, González-Pérez F, Udina E, del Valle J, Navarro X. (2016) Analysis of axonal growth in organotypic neural cultures. Protocol Exchange. doi: 10.1038/protex.2016.014.
2015
Comley LH, Allodi I*, Nichterwitz S, Nizzardo M, Simone C, Corti S, Hedlund E. (2015) Motor neurons with differential vulnerability to degeneration show distinct protein signatures in health and ALS. Neuroscience 16;291:216-29. *Co-first author.
2014
Allodi I, Mecollari V, González-Pérez F, Eggers R, Hoyng S, Verhaagen J, Navarro X, Udina E. (2014) Schwann cells transduced with a lentiviral vector encoding Fgf-2 promote motor neuron regulation following sciatic nerve injury. Glia 62(10):1736-46.
Allodi I, Hedlund E. (2014) Directed midbrain and spinal cord neurogenesis from pluripotent stem cells to model development and disease in a dish. Review. Front Neurosci. 20;8:109.
2013
Allodi I, Casals-Díaz L, Santos-Nogueira E, Gonzalez-Perez F, Navarro X, Udina E. (2013) FGF-2 low molecular weight selectively promotes neuritogenesis of motor neurons in vitro. Mol Neurobiology 47(2):770-81.
Torres-Espín A, Corona-Quintanilla DL, Forés J, Allodi I, González F, Udina E, Navarro X. (2013) Neuroprotection and axonal regeneration after lumbar ventral root avulsion by re-implantation and mesenchymal stem cells transplant combined therapy. Neurotherapeutics 10(2):354-68.
Auer M, Allodi I, Barham M, Udina E, Neiss WF, Navarro X, Klimaschewski L. (2013) C3 exoenzyme lacks effects on peripheral axon regeneration in vivo. J Peripher Nerv Syst 18(1):30-6.
2012
Allodi I, Udina E, Navarro X. (2012) Specificity of peripheral nerve regeneration: interaction at the axonal level. Review. Progress Neurobiol. 2012 Jul;98(1):16-37.
2011
Allodi I, Guzman-Lenis MS, Hernandez J, Navarro X, Udina E. (2011) In vitro comparison of motor and sensory neuron outgrowth in a 3D collagen matrix. J Neurosci Methods 15;198(1):53-61.
Udina E, Colbianchi S, Allodi I, Navarro X. (2011) Effects of activity-dependent strategies on regeneration and plasticity after peripheral nerve injuries. Review. Ann Anat. 193(4):347-53.
Lab members
| Name | Title | Job responsibilities | Image |
|---|---|---|---|
| Search in Name | Search in Title | Search in Job responsibilities | |
| Anna Villaume Stuckert | Master-Student | Allodi Lab |
|
| Beck Strohmer | Postdoc | Allodi Lab |
|
| Dana Blenda Ahlmark | Master-Student | Allodi Lab |
|
| Ilary Allodi | Guest Researcher | Allodi Lab |
|
