Research projects
Background: How is the brain supplied with food for thoughts?
And what goes wrong when we age, or suffer from a neurodegenerative disorder?
Normal brain function depends on preserved supply of glucose and oxygen and even minor deficits in control of the cerebral circulation lead to loss of brain function. The robust coupling between brain activity and cerebral blood flow (CBF), the so-called neurovascular coupling regulates the minor local CBF alterations that take place all the time. In the past, the dynamics of CBF control was based on an understanding of brain arterioles and on capillaries for exchange of substances between blood and brain. This notion has undergone an important change in recent years because it has been discovered that both arterioles and capillaries take part in 1) substance exchange and 2) cerebrovascular resistance.

Specifically, it was recently discovered that contractile cells called pericytes, which are attached to the capillaries, can regulate CBF at the capillary level. Understanding how microvascular blood flow and permeability is controlled will enable us to define and target mechanisms at the level of the smallest blood vessels, brain capillaries, which supply nerve cells with oxygen and glucose, i.e. brain energy. Failure of this energy supply causes neural tissue to degenerate, independently of the primary disease aetiology. The energy supply thus forms an attractive point of intervention for the development of disease modifying therapies for neurodegenerative diseases.
Drug transport across the brain endothelium forming the blood-brain barrier is another research challenge because of the low intrinsic permeability of the barrier to most solutes and the presence of active efflux transporters. However, it is of great importance to understand the mechanisms that control the access of active compounds across the BBB and to be able to study BBB permeability characteristics quantitatively and repeatedly is the same animal.
We use a combination of techniques to study neuroenergetics, cerebral blood flow and the signaling between cells of the neurovascular unit: electrophysiological techniques to record spike activity from single cells and local field potentials, electro-chemically recorded oxygen consumption, assessment of local blood flow with double-wavelength laser-Doppler flowmetry and laser speckle, opthogenetics and multi-photon microscopy for in vivo measurements in mice. We study both normal physiology, healthy aging and disease states such as stroke, cortical spreading depression and hypertension.
More details on our projects
Project participant
- Jonas Fordsman
Project description
Cortical Spreading Depression/Depolarization (CSD) is a depolarization wave in the cerebral grey matter, which in its mild form causes migraine, and in its severe forms may induce permanent damage of brain tissue. It is a remarkably complex event that involves dramatic changes in neural and vascular function.
CSD is an important mechanism for the expansion of ischemic or traumatic brain injury. Novel clinical studies have demonstrated numerous CSD episodes in up to 60 % of patients in the first week after traumatic brain injury, and in almost all patients with subarachnoid haemorrhage and malignant stroke. These discoveries indicate that CSD in cortical grey matter trigger prolonged hypoxia. CSD come in any forms like earthquakes on the Richter scale. The mild forms are associated with migraine while the severe forms are linked to the acutely injured human brain cortex where they kill brain cells by inducing increasingly big gaps between energy supply and use. The primary event, e.g. the stroke or the TBI kills the cell in the centre of the lesion but the brain creates so to speak further deterioration of brain tissue by single, repeated or even clustered depolarization waves that constrict blood vessels and use all the energy available. In the end the cells cannot repolarize and cells in the peri-ischemic region and cells die because of energy loss.
During and following CSD there are reports of a dramatic slowing of capillary red blood cell flow, flow arrest and even transient disappearance of red blood cells from capillary segments lasting as long as a minute. However, the studies carried out so far have been unable to document focal or diffuse capillary narrowing, compression or occlusion. The flow reduction in parenchymal arterioles and capillaries may precede vasoconstriction in larger pial arteries, but the principle site at which the cerebrovascular resistance increases is unclear. This is of critical relevance for an understanding of the role of CSD in the pathophysiology of stroke and other brain injury states.

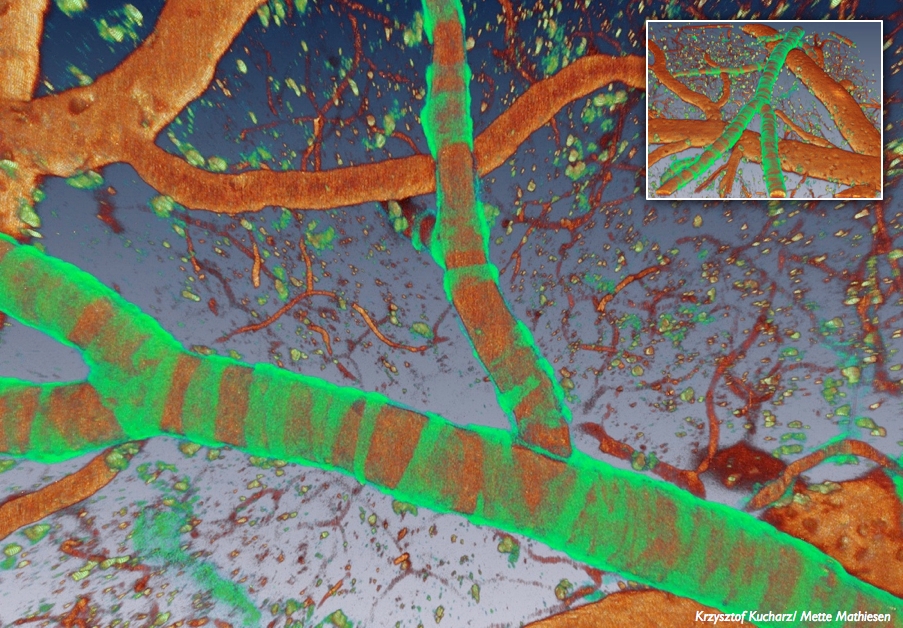
Two-photon image of mouse cortex in which a red fluorescent indicator is expressed in smooth muscle cells (large blood vessel) and in capillary pericytes (single cells on smaller vessels).
In our ongoing project we use mice with genetically expressed fluorescent indicators in vivo, to investigate the impact of CSD on the neurovascular unit and in particular the communication between cells at different levels of branching. We also use genetic mouse models of migraine with aura, Familial Hemiplegic Migraine type 1 (FHM1) and type 2 (FHM2) and a mouse model of stroke in which we evaluate the role of CSDs in the peri-ischemic region.
Recent publications from the group:
- Ayata C, Lauritzen M: Spreading Depression, Spreading Depolarizations, and the Cerebral Vasculature. Physiol Rev 2015;95:953-993.
- Kucharz K, Sondergaard Rasmussen I, Bach A, Stromgaard K, Lauritzen M. PSD-95 uncoupling from NMDA receptors by Tat-N-dimer ameliorates neuronal depolarisation in cortical spreading depression. J Cereb Blood Flow Metab. 2016 Apr 22. pii: 0271678X16645595. [Epub ahead of print] PMID: 27107027
- Khennouf L, Gesslein B, Lind BL, van den Maagdenberg AM, Lauritzen M. Activity-dependent calcium, oxygen, and vascular responses in a mouse model of familial hemiplegic migraine type 1. Ann Neurol. 2016;80(2):219-32.
Food for thought may be regulated by very delicate mechanisms
Project participants
- Barbara Lykke Lind
- Changsi Cai
- Reena Murmu Nielsen
- Stefan Andreas Zambach
- Aske Krogsgaard
- Bjørn Hald
- Søren Grubb
Project description
Changes in brain activity are accompanied by changes in brain metabolism and perfusion. This relationship is the basis of functional neuroimaging techniques where deviations in cerebral blood flow (CBF) or the blood oxygenation level-dependent (BOLD) signals are used to track brain activity.
Blood vessels filled with red fluorescent marker (TRITC-dextran) and green calcium indicator in smooth muscle cells.
Astrocytic end-feet are in close contact with blood vessels and may regulate stimulation-induced increases in cerebral blood flow, but this is a matter of debate. The main point of controversy is the timing and the size of the Ca2+ changes in astrocytic end-feet. In our previous work, we used organic dyes to demonstrate fast Ca2+ increases in astrocytic end-feet that were strongly correlated to the stimulation-induced rise in cerebral blood flow. In our current project we develop a new method for unbiased detection of rises of localized calcium activity with high specificity for astrocytes using organic fluorophores or astrocyte-specific, genetically encoded Ca2+ indicators
Another controversy is whether the major point of cerebrovascular resistance resides in arterioles or capillaries and what role pericytes play in local vascular control. Do pericytes the sense local metabolic needs?
In the past, it has been difficult to reliably identify capillary pericytes in living tissue. This problem has now been solved by the development of transgenic mice with fluorescent markers in pericytes. We use mice with pericytes expressing the fluorescent protein dsRed under control of the NG2 promoter allowing us to readily identify pericytes by two-photon microscopy in vivo. Experimental evidence supports a variety of functions for brain pericytes including formation, maintenance and regulation of the cerebral capillaries and control of the properties of the blood brain barrier (BBB). Recently, the lab has disclosed a new role for pericytes in living animals by demonstrating active regulation of blood flow in the capillaries. This was based on segmental alterations in capillary diameter in response to neural activity that concurred with the localization of pericyte cell bodies. These findings suggest that blood flow regulation not only takes place in arterioles (as previously believed) but that capillaries may constitute a major player. In ischemia, abnormal release of constricting molecules or defective release of dilators leads to pericytes constricting capillaries. This will lead to an augmented neuronal damage and is expected to produce dysfunction of the BBB. Similar pericyte malfunction may contribute to brain frailty in ageing due to loss of capillary blood flow control, but the mechanisms are incompletely understood.
Our current project develops experimental and analytical tools to study pericyte signaling in vivo. These tools are then used to study signaling within and between pericytes and the cells in the neurovascular unit. We use two-photon microscopy combined with electrophysiology, laser speckle and optogenetics. The number of pericytes decreases with age paralleling an increase in BBB permeability, and in disease states such as cerebral ischemia. Hence we study the blood flow regulation in both young healthy adults and in disease models and aging.
- Hall, C. N., C. Reynell, B. Gesslein, N. B. Hamilton, A. Mishra, B. A. Sutherland, F. M. O'Farrell, A. M. Buchan, M. Lauritzen and D. Attwell (2014). "Capillary pericytes regulate cerebral blood flow in health and disease." Nature 508(7494): 55-60.
- Lind BL, Jessen SB, Lonstrup M, Josephine C, Bonvento G, Lauritzen M. Fast Ca2+ responses in astrocyte end-feet and neurovascular coupling in mice. Glia. 2017.
- Jessen, S. B., A. Brazhe, B. L. Lind, C. Mathiesen, K. Thomsen, K. Jensen and M. Lauritzen (2015). "GABAA Receptor-Mediated Bidirectional Control of Synaptic Activity, Intracellular Ca2+, Cerebral Blood Flow, and Oxygen Consumption in Mouse Somatosensory Cortex In Vivo." Cereb Cortex 25(9): 2594-2609.
- Lind, B. L., A. R. Brazhe, S. B. Jessen, F. C. Tan and M. J. Lauritzen (2013). "Rapid stimulus-evoked astrocyte Ca2+ elevations and hemodynamic responses in mouse somatosensory cortex in vivo." Proc Natl Acad Sci U S A 110(48): E4678-4687.
Project participants
- Matilda Dahlqvist
- Kirsten Thomsen
- Alexey Brazhe
- Bjørn Hald
Project description
Population prognostics show that by the year 2030, 29% of the Danish population will be 60+ years compared to 24% today and that much of this increase will be due to increased numbers of persons 80 years of age or more. Similar aging populations are seen world-wide. With this shift in population demographics, there is also increased prevalence of diseases related to aging. Why is age a risk factor for sustained damage following slight head trauma in the elderly and what mechanisms in brain ageing makes the brain frail and predisposes to neurodegenerative diseases such as Alzheimer’s disease? That is the question.
Our first study showed that a concerted action among astrocytes in aged brains giving rise to astroglial calcium waves. These waves were abundant in old but not young brains and evoked small but repeated rises in oxygen consumption. This is one brain ageing mechanism with the potential to reduce brain's oxidative reserve capacity and thereby contribute to brain frailty.
Healthy brain aging is also characterized by reduced cerebral blood flow (CBF) and reduced responses to rises in neuronal activity – the supply of food for thought is restricted and even when the need goes up supply is at the borderline of being insufficient. Our second study reported that this was strongly correlated to reduced spontaneous and evoked gamma activity, which is strongly linked to perception, cognition and memory is humans as well as rodents. Gamma oscillations may drive the mechanisms responsible adjusting blood flow to brain activity, which decrease with age. In contrast, the coupling between metabolism and activity increase with age, despite reduced gamma oscillations in old animals. This could suggest that gamma activity and the interneurons that produce them are key players in healthy brain aging.
In our current project we use mice with parvalbumine (PV) positive interneurons that can be stimulated by light, so called optogenetic stimulation. This research project is believed to shed light on the importance of this particular type of interneuron in vascular and metabolic regulation in young and old mice.
- Thomsen K, Yokota T, Hasan-Olive MM, Sherazi N, Fakouri NB, Desler C, Regnell CE, Larsen S, Rasmussen LJ, Dela F, Bergersen LH, Lauritzen M. Initial brain aging: heterogeneity of mitochondrial size is associated with decline in complex I-linked respiration in cortex and hippocampus. Neurobiol Aging. 2017.
- Mathiesen C, Brazhe A, Thomsen K, Lauritzen M: Spontaneous calcium waves in Bergman glia increase with age and hypoxia and may reduce tissue oxygen. J Cereb Blood Flow Metab 2013;33:161-169.
- Brazhe A, Mathiesen C, Lauritzen M: Multiscale vision model highlights spontaneous glial calcium waves recorded by 2-photon imaging in brain tissue. Neuroimage 2013;68:192-202
- Jessen, S. B., C. Mathiesen, B. L. Lind and M. Lauritzen (2015). "Interneuron Deficit Associates Attenuated Network Synchronization to Mismatch of Energy Supply and Demand in Aging Mouse Brains." Cereb Cortex. 2015 Oct 29. pii: bhv261. [Epub ahead of print] PMID: 26514162
Project participants
Project description
Within the last 2 years we have gotten involved in blood-brain barrier (BBB) research, which is of great interest and of key importance for an understanding of the exchange of molecules across brain capillaries. Drug delivery to the central nervous system is a tremendous challenge. Drug transport across the brain endothelium forming the BBB is a particularly great challenge because of the low intrinsic permeability of the barrier to most solutes and the presence of active efflux transporters.
The Research Initiative on Brain Barriers and Drug Delivery (RIBBDD) was established as a network of five independent research units based at four Danish universities, Aalborg University (AAU), Aarhus University (AU), the Technical University of Denmark (DTU) and University of Copenhagen (UCPH). RIBBDD started its activities in March 2014 and is funded by the Lundbeck Foundation. The vision is to establish a cutting-edge research program that goes beyond the current possibilities of each group. The laboratories will work together to develop new research methodologies and approaches for studying and enforcing drug transport across the BBB, cellular communication in the brain, and regulation of the BBB.
Our work package is divided into six projects all focused on elucidating function and pathology of the NVU and the BBB. There is great interest in the mechanisms by which neurons, astrocytes and vascular cells interact, and how this interplay controls the properties of the BBB. Recent studies suggest that pericytes play an essential role for blood-central nervous system barriers and that the lack of pericytes or impairment of function leads to accumulation of toxic substances in the tissue and in turn cell death.
A main aim of our lab is to develop experimental and analytical tools to be able to study BBB properties at the level of single capillaries for big and small molecules and to develop tools for in vivo evaluation of BBB permeability and diffusion coefficients for relevant substances in normal physiology and under conditions with a disrupted BBB such as after stroke or chemical treatment of the BBB. Little is known with regard to signaling among pericytes and astrocytes in disease states, and the consequences of failed communication between cells of the neurovascular unit for BBB breakdown. Therefore a second aim is to outline if signaling among NVU cells, or changes in NVU communication pathways contribute to BBB breakdown in focal ischemia in mice.
There are as yet no publications available, but we expect that this will change in 2017.
Project participants
- Reena Murmu Nielsen
- Jonas Fordsmann
Project description
Cerebral Ischemic stroke is a leading neurological disorder that causes severe brain damage, disability and mortality. Despite decades of effort into developing effective therapies for stroke, the treatment strategies available are unable to prevent progression of damage cause by cerebral ischemic lesion (or stroke) in the acutely injured human brain. Moreover, neuroprotective drugs which, have been validated in preclinical models have failed to achieve desirable clinical benefits in clinical trials. Identifying new molecular pathways and mechanisms that underlie the progression of pathology in injured human brain post-stroke is vital in developing effective therapies to treat this disorder.
Recent studies suggest that restoring the integrity of the so called “Extended Neurovascular unit” is therapeutically vital for the successful treatment of ischemic stroke. The neurovascular unit is a functional and structurally interdependent multi-cellular complex, comprised of endothelial cells, the basal lamina, pericytes, astrocytes, and neurons. Dysfunction of the neurovascular unit is associated with a reduction in cerebral blood flow (CBF) and hypoxia ultimately leading to neuronal death. Studies have shown that in stroke neurovascular dysfunction prevails outside the ischemic core, in the penumbral area (the region that is immediately adjacent to the core with less severe blood perfusion deficits), however the underlying molecular mechanisms are not completely understood.
Astrocytes regulate neuronal function, synaptic plasticity and cerebral blood flow by elevations in Ca2+ concentrations in soma as well as in their cellular processes. Further, brain astrocytes use Ca2+ waves (ACW’s) as a means for communicating with each other and with other cell-types. Recent studies have suggested that the occurrence of spontaneous glial Ca2+ waves (ACW’s) was 20-fold higher in the cortex of aging brain as compared to the adult brain and that increased Ca2+ waves in astrocytes correlated with the reduction in resting brain oxygen tension suggesting a relationship between glial waves, brain energy homeostatic and pathology.
Laser speckle imaging of the brain before and after stroke. Healthy tissue (CBF >50 % of pre-stroke values) shown in gray scales. Peri-ischemic area shown in blue and ischemic core (CBF <20 % of pre-stroke values) in purple. Each panel corresponds to different times relative to stroke. Black scale bar = 1 mm.
In this study, we hypothesize that glial Ca2+ signals (both in the form of transients Ca2+ elevations and ACW’s) might represent the underlying mechanisms of damage in ischemic penumbra in stroke. By using state-of-the-art two-photon Ca2+ imaging and electrophysiology, we aim to examine astroglial Ca2+ signals and waves and its impact on neuronal function in a mouse model of stroke (middle cerebral occlusion model).
Project participant
Project description
The healthy brain needs fully functional intracellular organelles, which are distributed in even the smallest parts of the nerve cells. Activation of synaptic receptors during normal brain function triggers a number of processes that are essential for information processing. The majority of these signaling pathways converge on the endoplasmic reticulum (ER), which is of particular importance for basic neuronal function: neurotransmission and cell survival.
In vivo two-photon image of cortical neurons expressing enhanced green fluorescent protein targeted to ER lumen (EGFP-ER).
The key roles of ER in neurons are biosynthesis, Ca2+ store and signaling. The neuronal ER is functionally and structurally heterogeneous, and forms the single largest continuous organelle that extends from the nuclear envelope to subset of dendritic spines, and through the axon to presynaptic terminals. This organization allows long-distance trafficking of proteins in secretory pathway and cytosol-independent tunneling of ions between distal neuronal compartments. Intraluminal diffusion and equilibration of proteins and ions preserves the efficiency of protein folding machinery, facilitates neurotransmission and different forms of synaptic plasticity. In addition, the neuronal ER is an excitable organelle that conveys synaptically evoked regenerative Ca2+ waves that spread in dendrites, and can invade nuclear envelope. Often, the neuronal ER is conceptualized as a signal integrating organelle that couples spatially and temporally separated events in the cell i.e. synaptic activation with gene transcription. Thus, the alteration of ER continuity, even if transient would affect fundamental neuronal functions.
Yet, transgression from the continuous to discontinuous ER, in contrast to non-neuronal cells, is linked in neurons to apoptotic or necrotic cell death and the neuronal ER structure-function interplay is poorly understood. Furthermore, there is reportedly no longitudinal description of ER structural dynamics in the living brain.
Here, using two-photon imaging we investigate the ER morphology in intact brains in anesthetized mice. We developed real-time quantification of ER morphology dynamics and perform functional assessments of ER continuity in vivo. This is performed simultaneously with intracellular Ca2+ imaging and electrophysiological recordings of brain neuronal activity.
We describe an unusual property of ER rapidly responding to synaptic activation, with important implications to brain function in vivo. We characterize neuronal ER dynamics in physiology and in conditions that typically accompany ischemic stroke or brain trauma. Using different pharmacological approaches we determine the major signaling pathway that is involved in regulation of ER structural dynamics and suggest a new target for novel neuroprotective strategies.
We expect to present the results in 2017.
