CombiGene and Spark Therapeutics enter licensing agreement for gene therapy candidate CG01
The Swedish gene therapy company CombiGene AB which was co-founded by Assoc. Prof David Woldbye at the Department of Neuroscience, Univ of CPH has just entered into a global collaboration and licensing agreement for the gene therapy candidate CG01 with Spark Therapeutics, a member of the Roche Group and a fully integrated, commercial gene therapy company.
Spark Therapeutics is known in Denmark for developing the gene therapy product Luxturna® (voretigen neparvovec) for treating blindness associated with Leber’s amaurosis. The agreement provides Spark with the exclusive world-wide license to develop, manufacture and commercialize CG01. CombiGene will continue to execute certain aspects of the preclinical program in collaboration with Spark. Under the terms of agreement, CombiGene is eligible to receive up to $328.5 million excluding royalties, with $8.5 million upon signing, up to $50 million at preclinical and clinical milestones.
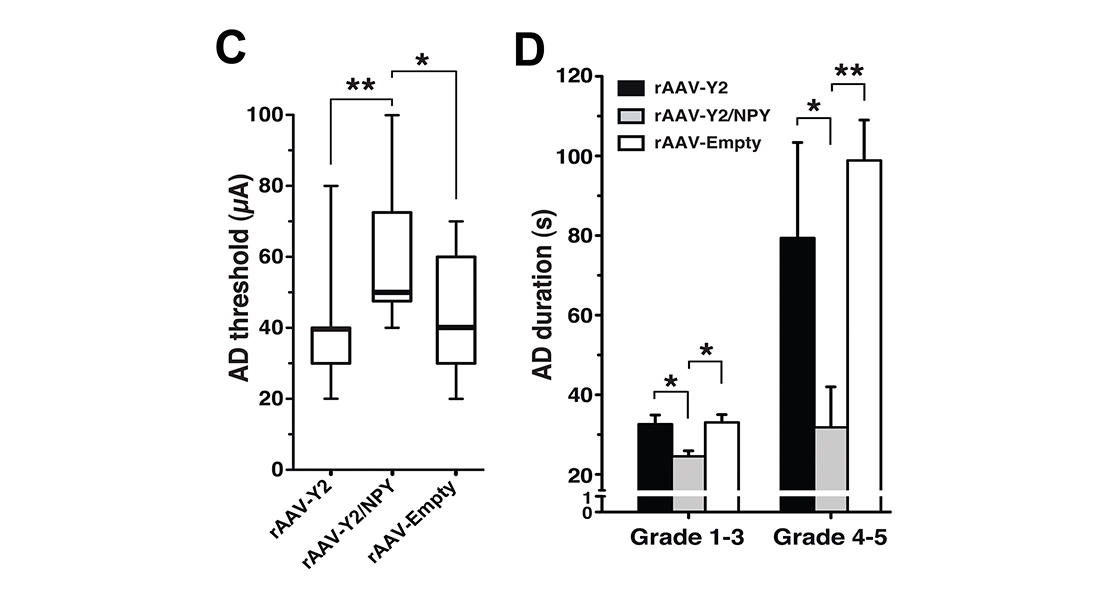
“This is a great day for CombiGene and for me”, says Assoc Prof Woldbye. This is the culmination of my preclinical work dating back as long as 1996 where it was shown that neuropeptide Y exerts seizure-suppressant effects. In a long-term collaboration with Professor Merab Kokaia at Lund University, Sweden, we later showed that vector-mediated overexpression of NPY and its receptor Y2 could be the way forward to treat epileptic seizures via a novel gene therapy. With the deal with Spark we now have ensured that this treatment can be tested in the clinic for temporal lobe epilepsy. Since many of these patients are drug resistant, there is a great need for developing novel treatments. “It is my hope that with this deal, we can now get a chance to help these epilepsy patients”.