News from the MacAulay Lab
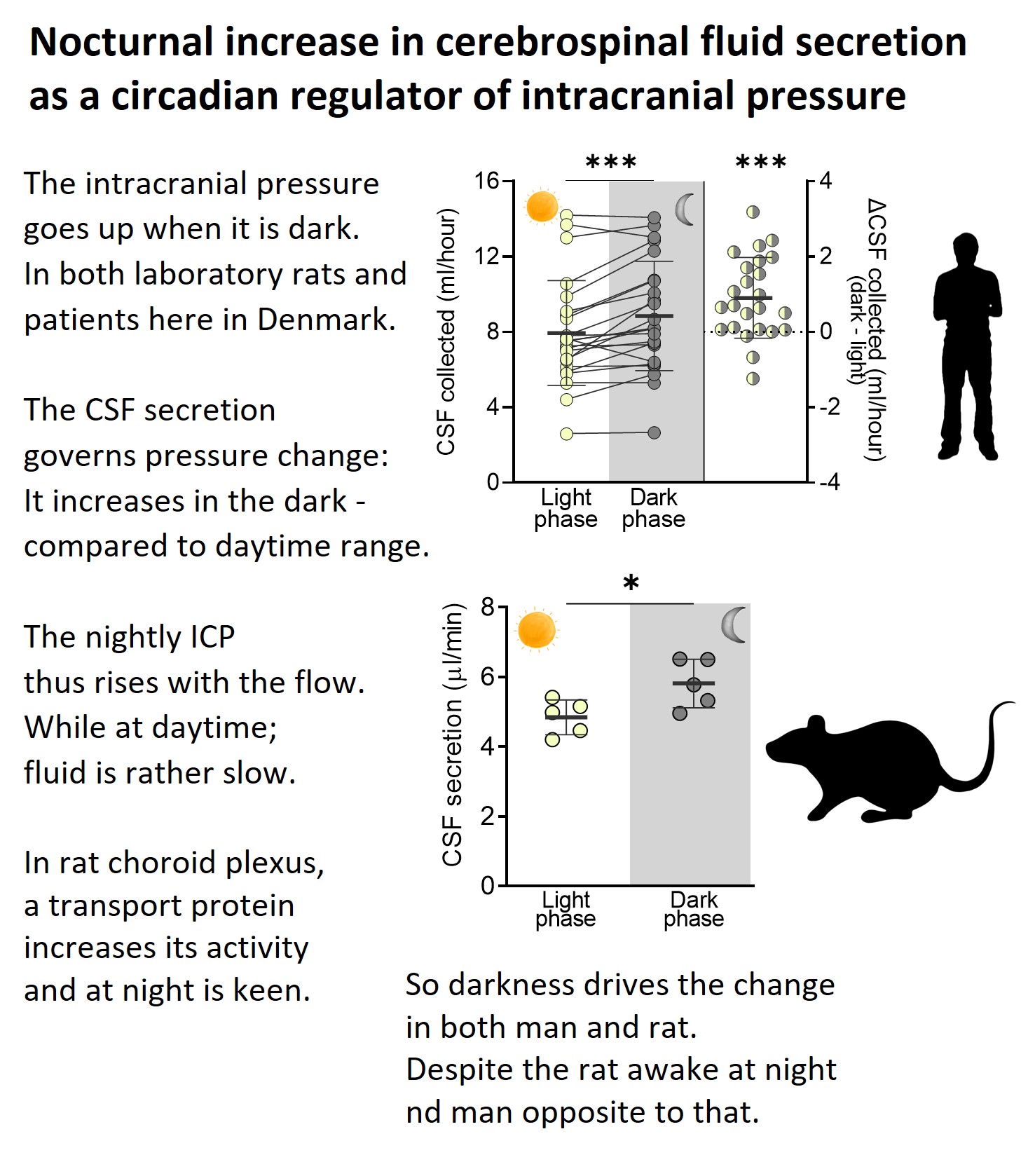
The intracranial pressure is elevated at night, in part due to a nightly increase in the rate of brain fluid secretion.
Oddly enough – we observed this diurnal pattern in both humans and rats, despite the latter being a nocturnal species and us being day-active. So here goes the search for the molecular driver......... To be continued.
Thanks to my wonderful research group Annette, Beatriche, Dagne, and Søren for their devotion to this project with all the long nights doing experiments, to our clinical collaborators Kirsten Møller and Markus H. Olsen, and to Lundbeckfonden for their support of this project with an Ascending Investigator grant. Highly appreciated.
To read the full study: https://rdcu.be/dffN5
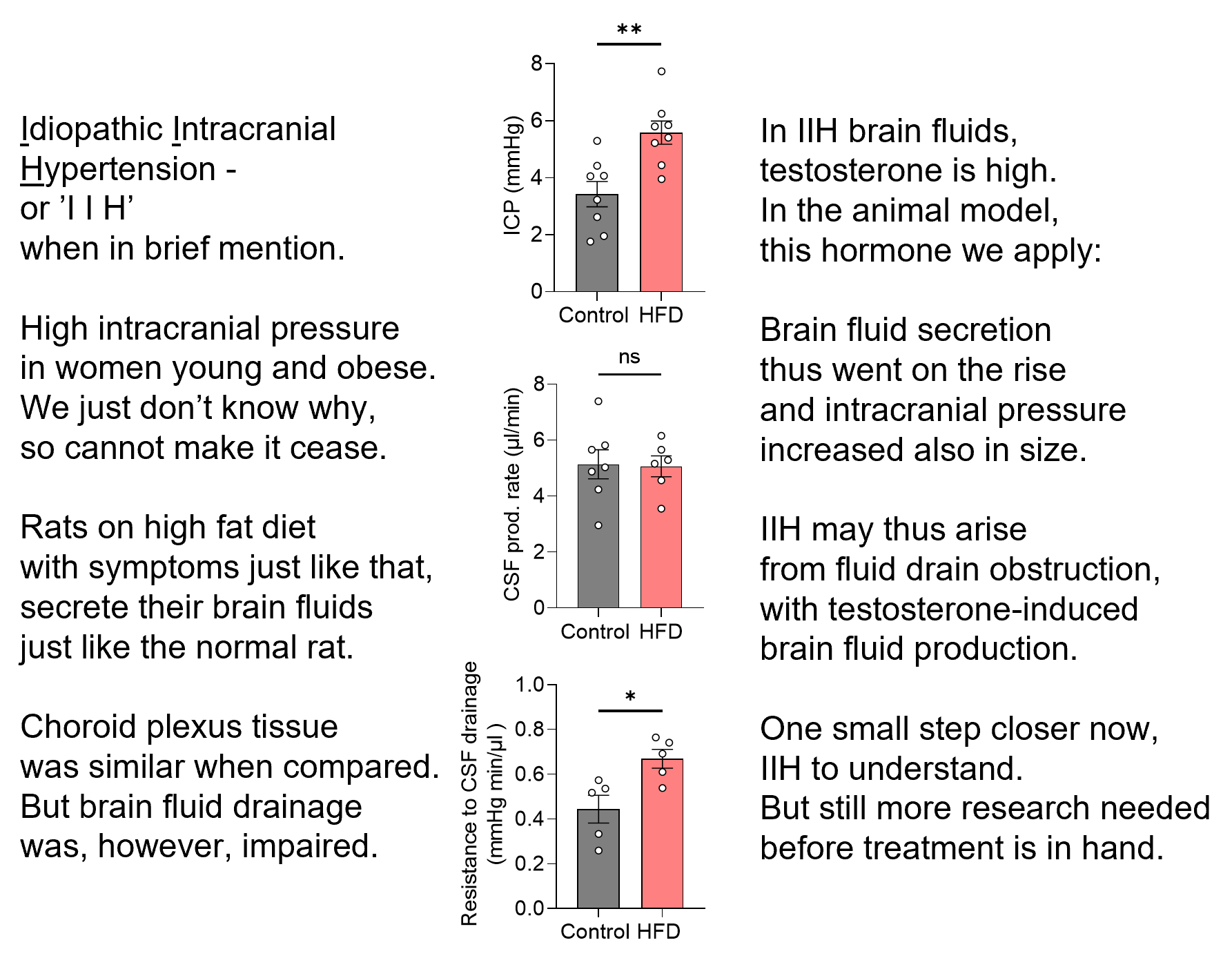
Idiopathic intracranial hypertension – IIH. That means elevated pressure inside the skull of unknown origin.
- Who: young obese women.
- What: severe headache and potential loss of vision
- How many: on the rise with increased prevalence of obesity
- Why: we don’t know
- How: we don’t know
- Treatment: lacking (other than weight loss)
To get to the ‘why’ and ‘how’ – with a vision to obtain the ‘treatment’, we fattened female rats and performed the brain fluid dynamics measurements that one cannot do on humans.
As you can read on verse on the image (or in the published paper: https://rdcu.be/deJfi), these rats did get elevated intracranial pressure like the patients. The brain fluid drainage was reduced (=think kitchen drain was partly clogged), but the brain fluid production was undisturbed (=think kitchen tap fully functional). Until......
The rats were treated with the testosterone that is detected at high levels in the patients’ brain fluid: the brain fluid was produced at a higher rate (=think the kitchen tap was turned up) – leading to an elevation of the intracranial pressure (=think full kitchen sink).
We must understand brain fluid dynamics in physiology to be able to control it in pathology – and we will. One step at a time. With the clear vision to provide a complementary approach to that of invasive neurosurgery to benefit the many patients experiencing brain fluid accumulation and elevated intracranial pressure in association with a variety of different brain and metabolic pathologies.
Thanks to the Lundbeck Foundation for supporting this study.
Researchers from the University of Copenhagen have discovered that fluid does not necessarily enter the brain the way one thought. According to one of the researchers behind the study, the result may lead to fewer major brain operations.
Read the full article here.
Now we are one (small) step closer to our vision of obtaining medicine for conditions of elevated brain water content and thus elevated intracranial pressure.
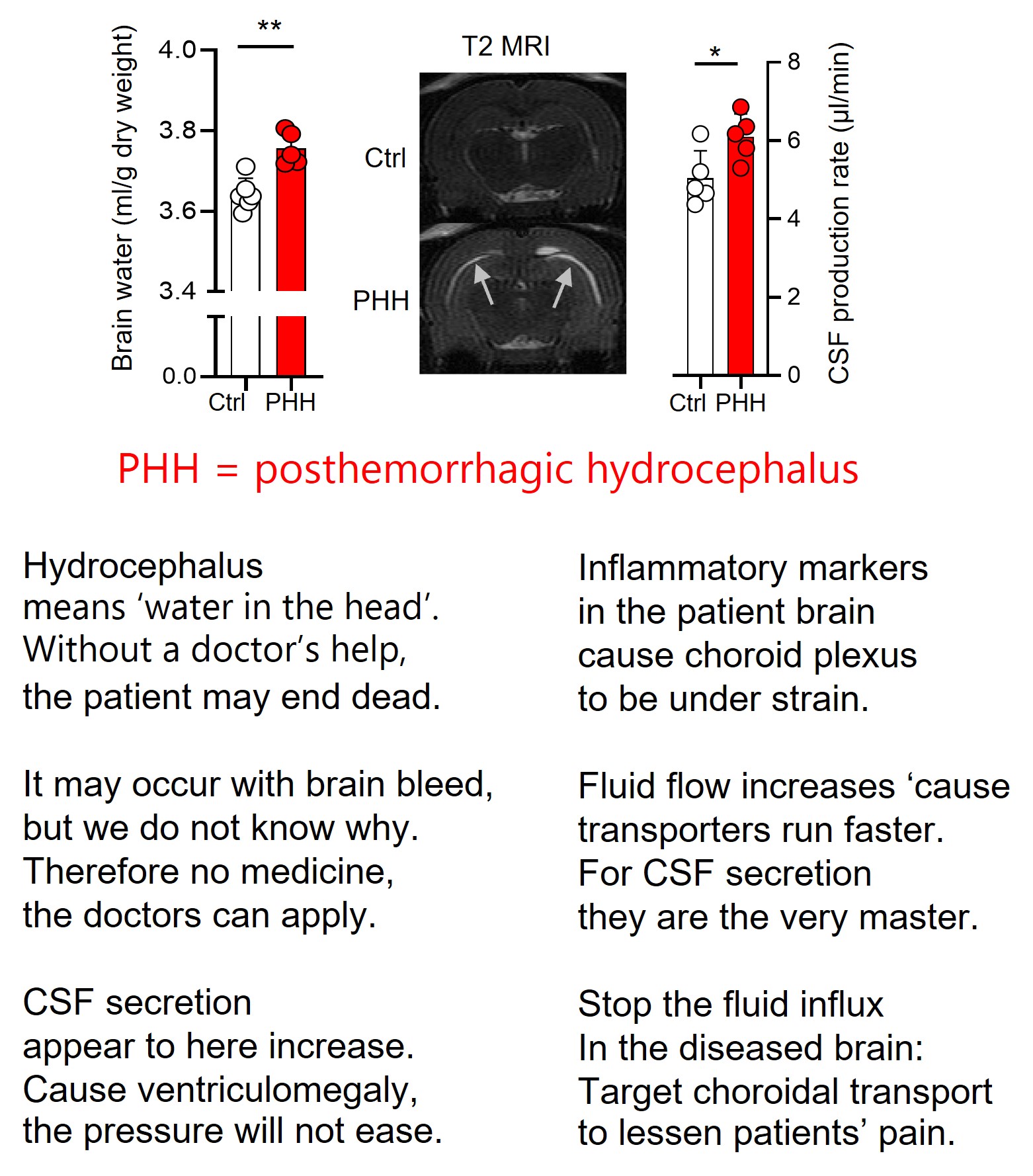
Hydrocephalus (too much water in the head) occurs as a consequence of brain bleeds – either in neonatal babies or in the general population following trauma, ruptured aneurisms, or the like.
It has generally been assumed that the water accumulation occurred following haemorrhage-mediated blockage of the brain fluid outflow. But here we demonstrate that some fraction of the fluid build-up originate from hypersecretion of the brain fluid that surrounds our brain and fills the central cavities.
The inflammatory conditions arising with the hemorrhagic event cause hyper-activity of the transporters in choroid plexus – and thereby elevate brain fluid secretion (see verses in the post or find the full text link in commentarys, if you are interested).
Transporter = protein = potential pharmacological target. We do need more – and better - of those! …..and will keep looking!
Thanks to all my wonderful co-workers @ and thanks to IMK Almene Fond and @novo for their support to this study.
An increasing number of elderly patients are diagnosed with a form of hydrocephalus that does not appear to cause elevated intracranial pressure – therefore the ‘normal pressure’ hydrocephalus. It comes across as an oxymoron (a useful word meaning ‘apparently contradictory terms that appear in conjunction’) that one can have enlarged ventricles an no elevation of pressure. ‘Idiopathic’ means that the cause is unknown.
We tested the hypothesis that this patient group has elevated osmolarity of their brain fluid (cerebrospinal fluid = CSF) – which they did not! (read the short summary on verse or find the article here).
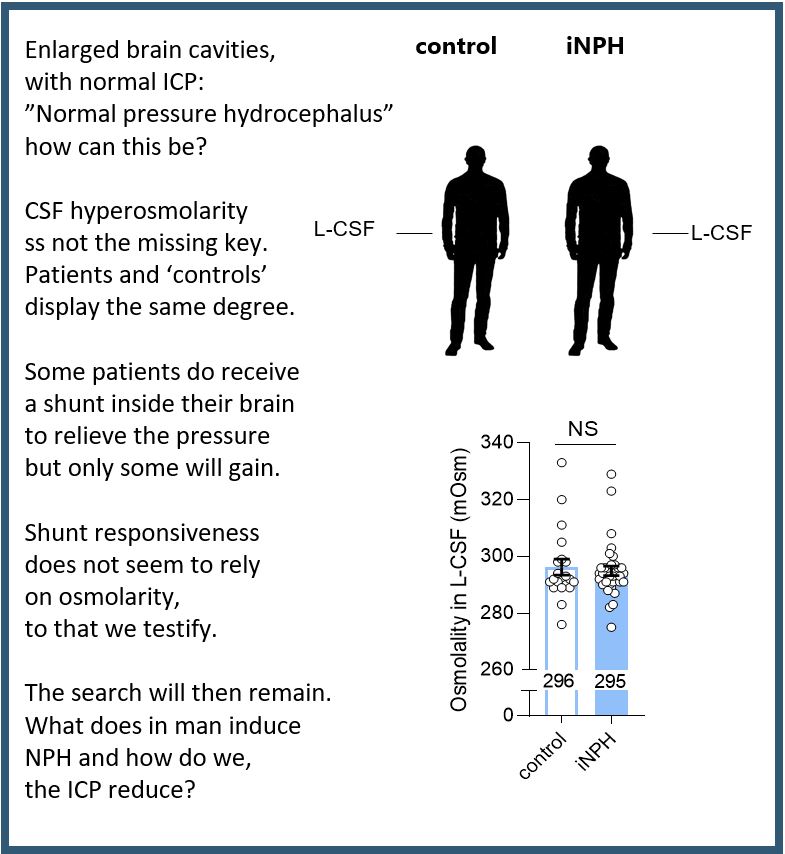
So the search continues. We need to know how the disease arises to be able to target a precise mechanism in future treatment options.
Thanks to all the patients who permitted us to use their cerebrospinal fluid that was sampled during the diagnostic work up and thanks to Novo Nordisk foundation for

Great to see all our wonderful NeuroGrads F2F again for the ‘fun part’ of our annual NeuroGrad Winter School. Thanks to 2021 Brain Prize winner Jes Olesen for taking us through his path from PhD student to winner of the most prestigious brain research prize, to Jan Hellesøe for showing us a different perspective of ‘fiddling with the brain’ (compared to patient/bench work), and thanks to all students and supervisors participating.
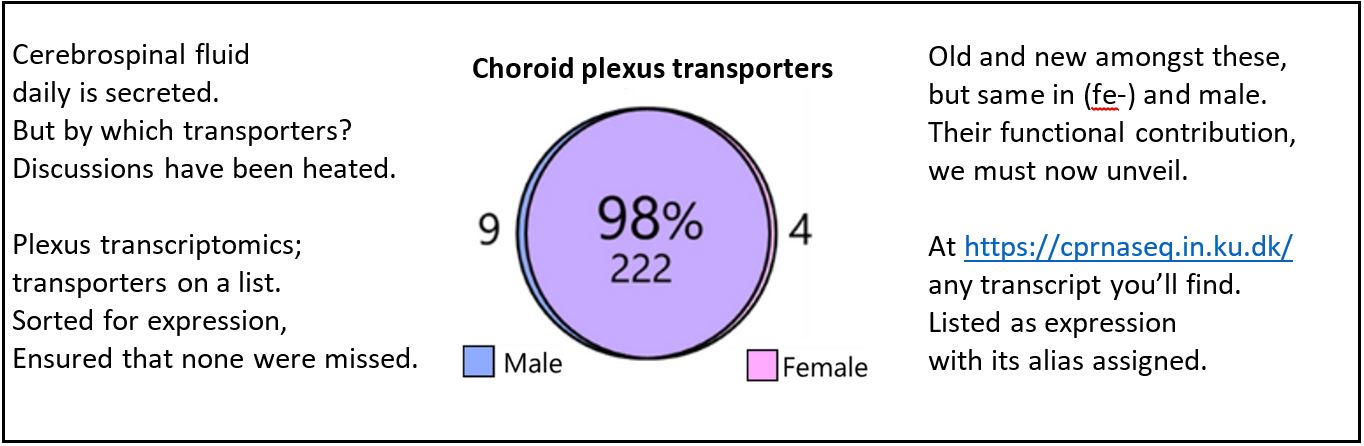
Crazy – isn’t it? A small tissue, with the complicated name choroid plexus, resides deep in our brains and secretes the 500 ml of fluid that daily enters our brain. But we know little about the transporters expressed in this tissue.
Here we identify, sort, and list the transporters, channels, receptors, and signaling molecules expressed in our very favorite tissue. Check out whether your favorite protein is amongst these either in the paper or in the associated database.
Great work Søren Norge Andreassen – and colleagues Trine L. Toft, Jonathan H. Wardman, and Rene Villadsen – and thanks to the Lundbeck Foundation for funding the project.
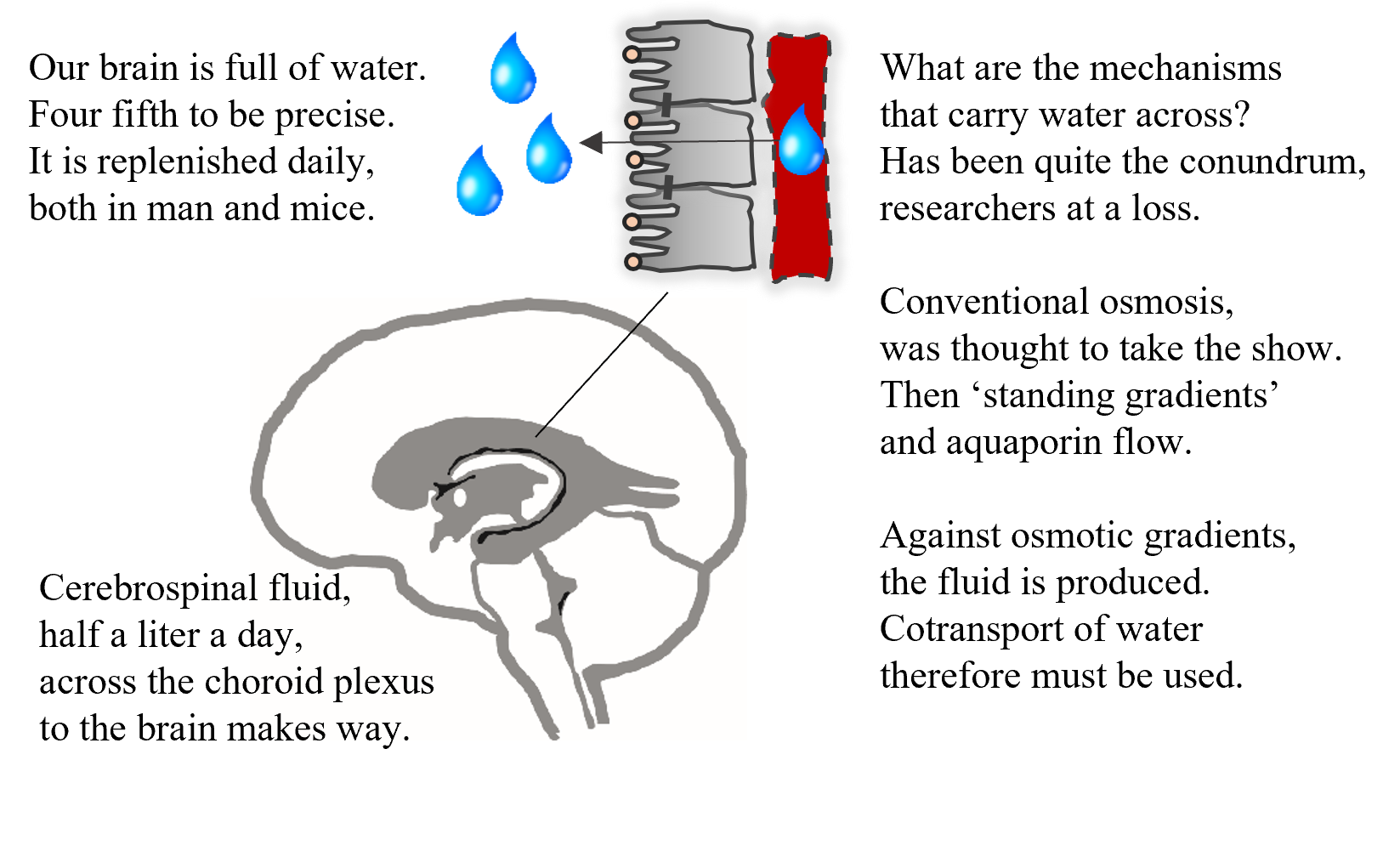
We have known for centuries that our brains are full of water – which was at some point in time even considered to contain our soul. The brain water is replenished at a rate of 500 ml water daily. But we barely understand how!
Since the stone age, surgeons have relieved elevated intracranial pressure (too much water in the brain) by cutting a hole in the skull. The level of refinement has obviously improved over the centuries, but the principle is the same. Crazy – isn’t it?
We must resolve the molecular mechanisms of how water enters into the brain – in order to therapeutically slow down these processes in pathologies involving too much brain water and speed them up in those involving too little brain water.
For those interested in the finer details of brain secretion, please click here. For those who just want a quick glimpse into the fascinating world of brain water transport, read the poem on the image.
Thanks to all the funding agencies who support our work on BrainH2O – absolutely essential for our path towards pharmacological treatment of these clinically challenging pathologies.
Traumatic brain injury and brain hemorrhage can lead to the brain water accumulation called hydrocephalus. The subsequent elevation in brain pressure can be fatal if left untreated. But we do not know how and why it happens and we have no efficient pharmacological treatment.
We are excited to share the molecular coupling between brain bleeding and brain water accumulation – either on verse here or on BioRxiv (find link in commentaries below). We hope this first stepping stone can bring us closer to medical treatment of this condition.
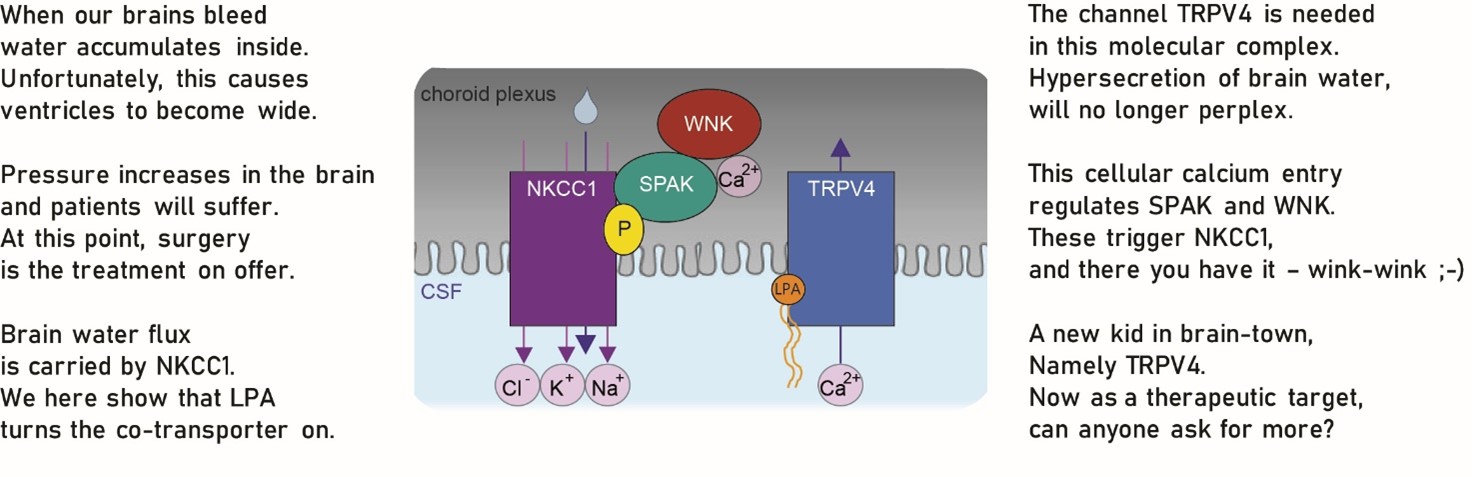
Congratulations to Trine for her accomplishment with this study and thanks to all our collaborators. The generous funding from LBF and NNF is highly appreciated
To manage intracranial pressure in pathology, we must understand brain fluid dynamics in physiology

Finally – a F2F conference!
Thanks to all the participants at the BrainH2O symposium in Copenhagen this past week. It was wonderful to see colleagues and friends from around the world again – and to share the science that we envision will help diagnose and treat the patients with disturbed brain fluid balance.
Thanks to the Novo Nordic Foundation and the graduate school at SUND for their support.
My review of molecular mechanisms governing brain water transport is now out in Nature Review Neuroscience (https://rdcu.be/cixTK). Or, if you prefer to acquire such knowledge on verse, then here you go. Enjoy. :)
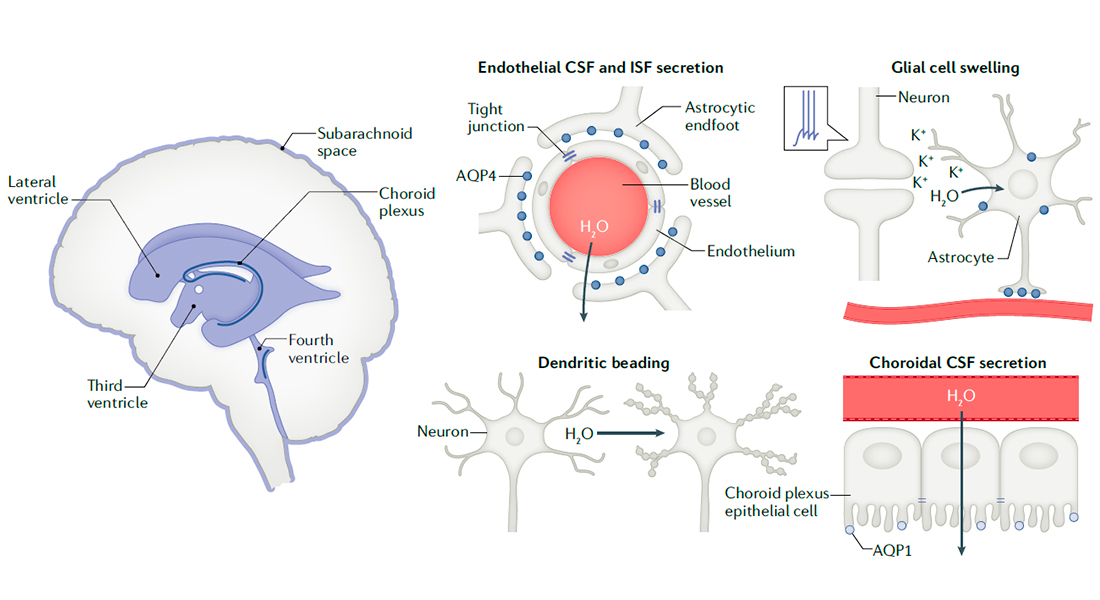
MacAulay 2021, Nat Rev Neuro
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
We have known about cerebrospinal fluid for centuries, and yet we know very little about how this brain fluid is generated. Not even the very basics of which direction the involved proteins run, can we agree on. Go Figure!
The water transporting Na+/K+/2Cl- cotransporter, NKCC1, secretes approximately half of the cerebrospinal fluid. In a @Journal of Physiology CrossTalk, we present evidence for NKCC1 transporting electrolytes and water from the cerebrospinal fluid-secreting tissue and into the brain.
See the CrossTalk ‘proposal’, ‘the opposing view’, the ‘rebuttal’, and our ‘rebuttal’
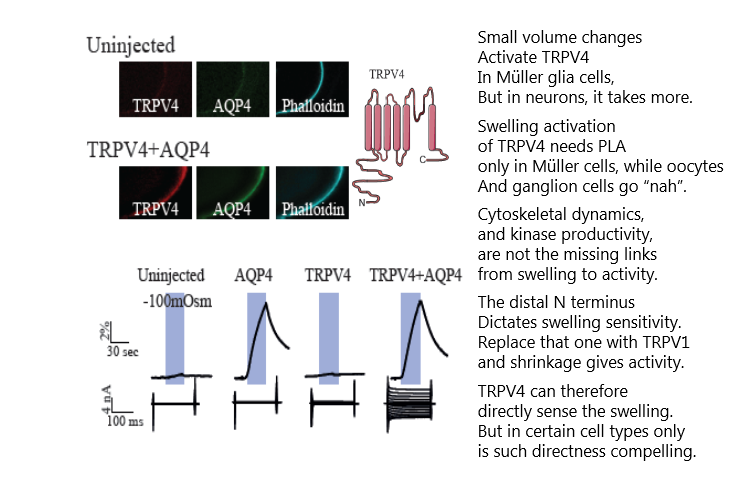
Research teams across the world have struggled to find an efficient and specific inhibitor for the brain water channel, aquaporin 4 (AQP4). Now it is here! The MacAulay lab demonstrates TGN-020 as such an isoform-specific pharmacological tool, with which the research community can test the plethora of proposed roles for AQP4 in brain (patho)physiology. For a start, this publication in #GLIA, cements that AQP4 and passive osmotic water permeability is not required for activity-evoked glia cell swelling.
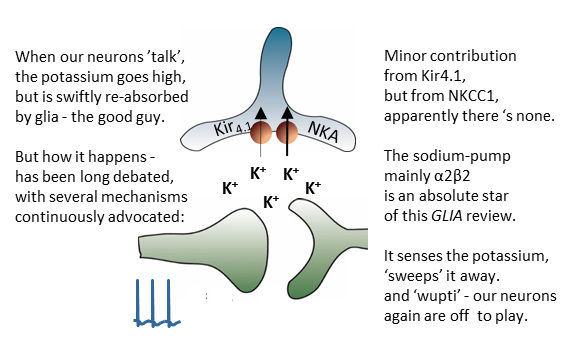 All neuroscientists are well aware that neuronal signaling associates with K+ release. While we have known for nearly a century that this K+ is swiftly removed from the extracellular space to ensure continuous neuronal activity, the mechanisms by which this crucial (patho)physiological event occurs have remained partly unresolved and highly controversial. This review article from the MacAulay laboratory, recently published in the journal GLIA, condenses the research from the past century and provides a comprehensive introduction to the topic.
All neuroscientists are well aware that neuronal signaling associates with K+ release. While we have known for nearly a century that this K+ is swiftly removed from the extracellular space to ensure continuous neuronal activity, the mechanisms by which this crucial (patho)physiological event occurs have remained partly unresolved and highly controversial. This review article from the MacAulay laboratory, recently published in the journal GLIA, condenses the research from the past century and provides a comprehensive introduction to the topic.
Thanks to all those joining me for an afternoon of brain water transport as I defended my Doctor dissertation to obtain the academic title of ’Doctor of Medical Sciences’ at University of Copenhagen (an academic degree above that of the PhD degree).
A special thanks to my two overseas opponents (Professor O’Donnell, UC Davis and Professor MacVicar, UBC) and to the Chairs of the evaluation committee (Professor Lauritzen) and of the defense ceremony (Professor Bräuner-Osborne) – and to all those of you who continued the festive celebration in the evening.
It was a wonderful experience and I highly appreciate participation of so many wonderful people.
Neuroscience graduate students at University of Copenhagen, the NeuroGrads, share their science in a string of amazing poster and oral presentations at the NeuroGrad Winter School 2020. Our excellent young scientists were joined by BrainPrize winner Professor Hugues Chabriat and Impostor Syndrome expert Dr. Valerie Young.
Thanks to the Lundbeck Foundation and the PhD school at Health and Medical Sciences, University of Copenhagen for sponsoring the event and to all participating NeuroGrads for the marvelous presentations.
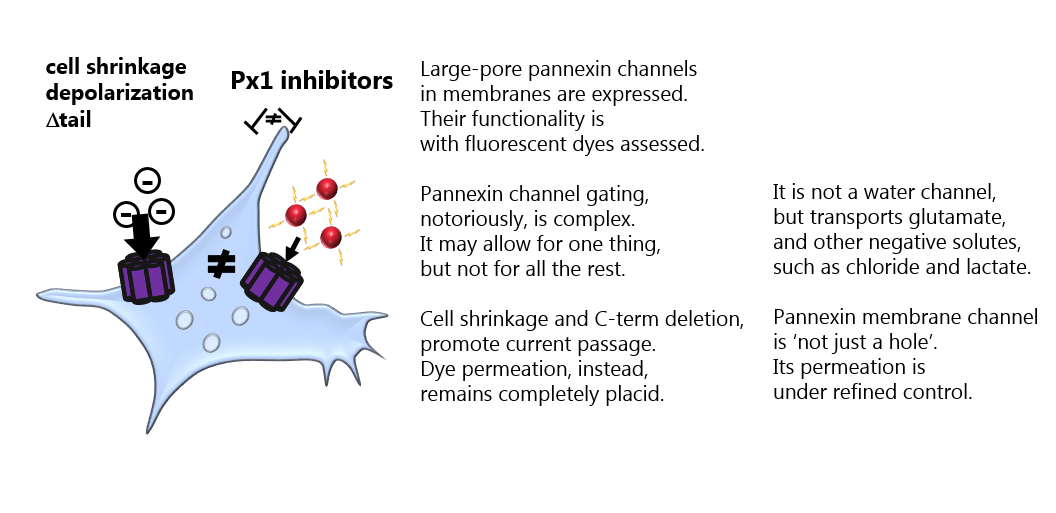
It has remained elusive what opens the Pannexin 1 large pore channel – and what permeates the channel when open. In this study from the MacAulay laboratory, we – by rigorous and comparative experiments in different cell types – demonstrate that an open channel is not just a hole that allows all molecules of a given size to enter. Pannexin 1, like its cousins the connexions hemichannels, is indeed able to act as a gated and selective channel. See publication and read the commentary in Journal of Physiology.
 It has been highly controversial in the connexin field what can permeate an open Cx43 hemichannel. The MacAulay laboratory publishes a study in Journal of Biological Chemistry on the structural determinants dictating that an open Cx43 hemichannel does not allow atomic ions to permeate: No Cx43 hemichannel-mediated ion conductance.
It has been highly controversial in the connexin field what can permeate an open Cx43 hemichannel. The MacAulay laboratory publishes a study in Journal of Biological Chemistry on the structural determinants dictating that an open Cx43 hemichannel does not allow atomic ions to permeate: No Cx43 hemichannel-mediated ion conductance.
‘No more brain surgery’ is the dream for children with hydrocephalus, who undergo brain surgery when their shunt repeatedly fails.
Thanks to the Hydrocephalus association for hosting us researchers at their excellent workshop in St. Louis, MO earlier this week: ‘Driving Common Pathways: Extending Insights from Posthemorrhagic Hydrocephalus’.
It was inspiring to meet the brave and optimistic parents of children with hydrocephalus at your fundraising event – as well as so many great scientists and neurosurgeons working tirelessly towards understanding the etiology of this condition and find a cure!
No more brain surgery! Let’s do this!
28-10-2019: Professor Nanna MacAulay receives the Lundbeck Foundation's Ascending Investigator grant

Professor Nanna MacAulay from Department of Neuroscience receives the Lundbeck Foundation’s Ascending Investigator Grant (5 mio. DKK) to determine the molecular mechanisms underlying sleep-induced regulation of the cerebrospinal fluid secretion.
The project has received10 mio. DKK from the Lundbeck Foundation’s thematic call: ‘What causes brain diseases’.
The MacAulay Laboratory is very excited to initiate this translational collaboration with clinicians Professor Rigmor H. Jensen, the headache center, Glostrup-Rigshospitalet, DK and Professor Alexandra Sinclair, Birmingham University, UK, who are both leading experts in the clinical aspects of this puzzling disease where young, obese females experience debilitating elevations in intracranial pressure. With an additional experimental ‘arm’ governed by transcriptomics expert Associate Professor Tune H. Pers and metobolomics expert Associate Professor Matthew Gillum, both from Novo Nordic Center for Basic Metabolic Research, University of Copenhagen, we will resolve the molecular mechanisms signifying the etiology of this disease - with the vision to create future pharmacological therapy to aid this growing patient group, for which no efficient treatment currently exists.
Thanks to the Lundbeck Foundation for their generous support of our research!
The MacAulay laboratory’s recent discovery of a role for cotransporters in brain fluid dynamics was covered in an issue of Newsweek, see article. The study revealed a novel form of fluid transport underlying the elusive formation of half a liter of brain water each day in the adult human. With this new finding, researchers have a first molecular handle to initiate rational pharmacological targeting of the brain fluid secretory machinery in diseases with disturbed brain water dynamics and disabling elevated intracranial pressure, such as hydrocephalus, stroke, traumatic brain injury, and brain tumors. The MacAulay laboratory is committed to resolving the intricate mechanisms and regulatory pathways governing our brain’s fluid management.
Thanks to the funding agencies that supported the research underlying these important findings (i.e. the Novo Nordic Foundation, the Independent Research Fund Denmark, Thorberg’s Foundation)